Use of Antibody–Drug Conjugates in the Early Setting of Breast Cancer
Authors: Chrysanthi Koukoutzeli, Dario Trapani, Liliana Ascione, Elias Kotteas, Antonio Marra, Carmen Criscitiello, Giuseppe Curigliano
Published in: Clinical Medicine Insights: Oncology, June 2024
Introduction
Antibody-drug conjugates (ADCs) represent a significant advancement in targeted cancer therapy, particularly in breast cancer treatment. Designed to deliver cytotoxic agents directly to cancer cells, ADCs consist of a monoclonal antibody (mAb) linked to a cytotoxic drug. This approach allows for the precise targeting of cancer cells with limited toxicity to healthy tissues. Currently, Ado-trastuzumab emtansine is the only ADC approved for early breast cancer, while several ADCs are approved for metastatic cases. The potential of ADCs in early-stage breast cancer is under investigation, promising enhanced therapeutic options through precision medicine.
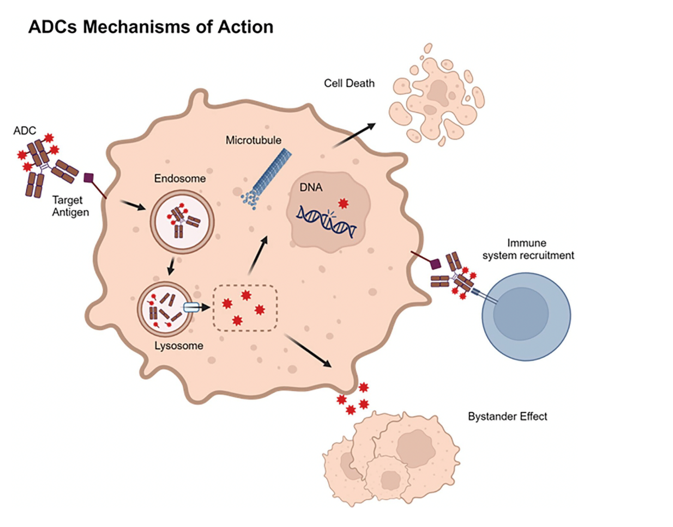
Figure Caption for Mechanism of ADC Action
ADCs bind to target antigens on cancer cells, forming ADC–antigen complexes that enter cells via endocytosis. Once inside, ADCs release cytotoxic payloads in lysosomes, leading to cell death by damaging DNA, disrupting microtubules, or other pathways. The payloads can also induce a “bystander effect,” impacting nearby non-targeted cells, and may activate the immune system to bolster anticancer effects.
Study focus
This narrative review assesses the role of ADCs in early breast cancer treatment, focusing on different ADC structures, their targets, and ongoing clinical trials. The article examines specific ADC agents, including LIV1-targeting ADCs and HER2/3-targeting ADCs, alongside Trop-2 ADCs like sacituzumab govitecan. ADCs were analyzed based on their drug-to-antibody ratio (DAR), linker stability, and payload effectiveness, considering their effectiveness in targeting tumor antigens and minimizing off-target toxicity. Clinical trials assessing the therapeutic effects and resistance mechanisms associated with ADCs provide insights into ADC safety profiles and efficacy in early breast cancer.
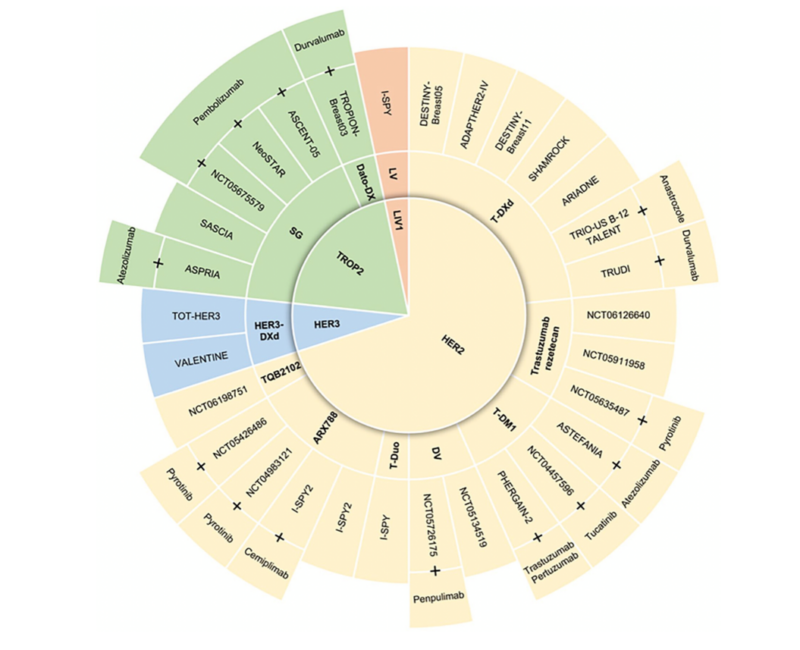
This Figure presents an overview of clinical trials involving ADCs in the early setting of breast cancer, highlighting the diversity of targets such as HER2, HER3, LIV1, and Trop-2, with ADCs including datopotamab deruxtecan (Dato-DXd), disitamab vedotin (DV), patritumab deruxtecan (HER3-DXd), ladiratuzumab vedotin (LV), and sacituzumab govitecan (SG), among others.
Key Highlights
- Targeting and Delivery: ADCs leverage monoclonal antibodies to target cancer cell antigens and deliver cytotoxic payloads, allowing for both direct cancer cell killing and a “bystander effect” on neighboring cells.
- Clinical Impact: ADCs like T-DM1 and T-DXd have shown promising results in metastatic breast cancer and are now being evaluated in early settings. The trials aim to reduce treatment toxicity while enhancing patient outcomes.
- Innovative ADCs in Early Trials: ADCs such as trastuzumab rezetecan and ARX788 are being explored for enhanced delivery and stability. Notably, T-DXd demonstrates efficacy in HER2-low tumors, expanding treatment options.
- Combination Therapy: ADCs combined with immunotherapy, like T-DM1 with atezolizumab, show promise for increasing efficacy by boosting the immune response against tumors.
Key Takeaways
- ADCs are an evolving class in oncology, proving beneficial in breast cancer by combining targeted mAb technology with potent cytotoxic payloads.
- Precision medicine paradigms and treatment de-escalation are potential future applications, allowing for minimized toxicity and enhanced patient-specific outcomes.
- Challenges, such as off-target toxicity, require continued optimization, including selecting appropriate target antigens and refining ADC components to increase tumor selectivity and minimize side effects.
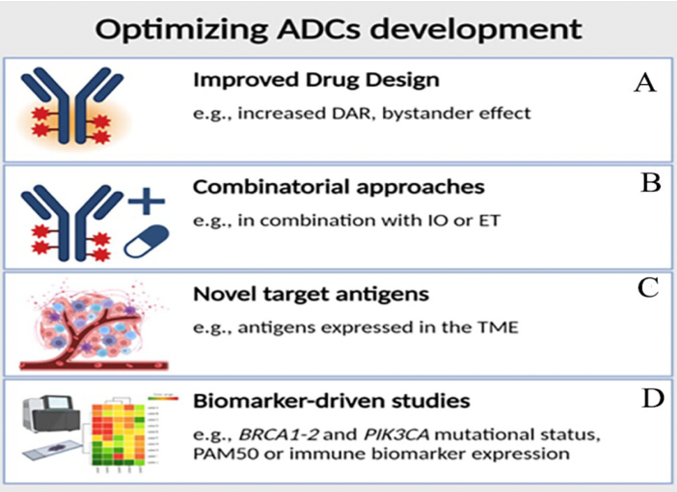
This figure highlights strategies to enhance antibody-drug conjugate (ADC) efficacy: (A) Increasing drug-to-antibody ratio (DAR) and utilizing strong bystander effects; (B) Selecting optimal ADCs and combination partners; (C) Targeting components of the neovascular system to reduce mutational escape; (D) Employing biomarker-enhanced studies (e.g., PAM50, PIK3CA, BRCA) to personalize ADC therapies. ADC indicates antibody-drug conjugate; DAR, drug-to-antibody ratio; TME, tumor microenvironment.
Future Directions
The narrative concludes with a call for further research into the mechanisms of resistance to ADCs and the development of next-generation conjugates. Emphasizing the importance of identifying suitable biomarkers for patient stratification, the review advocates for integrating ADCs into standard treatment protocols for early breast cancer, ultimately aiming to enhance efficacy while minimizing toxicity.


