Piotr Wysocki recently shared on LinkedIn:
“Toni Choueri et al published the results of a phase III LITESPARK-005 study comparing belzutifan with everolimus in advanced clear-cell renal-cell carcinoma (ccRCC) patients who had previously received immune checkpoint and antiangiogenic therapies. Belzutifan (MK-6482) is a potent small-molecule inhibitor of HIF-2α that prevents heterodimerization with HIF-1β into an active transcription factor that plays an essential role in the biology of ccRCC.
The open-label trial enrolled 746 participants who were randomly assigned to receive either belzutifan (120 mg daily) or everolimus (10 mg daily) in a 1:1 ratio. The primary endpoints were progression-free survival (PFS) and overall survival (OS). A key secondary endpoint was the objective response rate (ORR), defined as the occurrence of a confirmed complete or partial response.
Results:
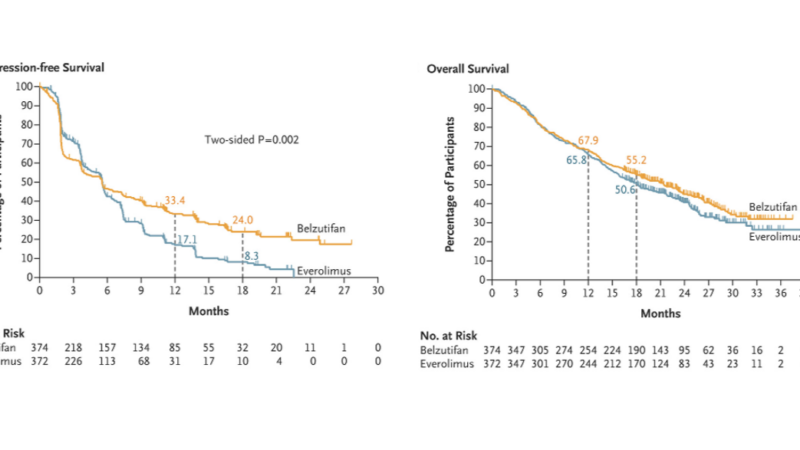
- Progression-Free Survival: At the first interim analysis, with a median follow-up of 18.4 months, the median PFS was 5.6 months in both treatment groups. However, at 18 months, 24.0% of patients in the belzutifan group were alive and free from disease progression, compared to 8.3% in the everolimus group (P=0.002).
- Objective Response Rate: The ORR was significantly higher in the belzutifan group, with 21.9% of participants achieving a confirmed response, compared to 3.5% in the everolimus group (P<0.001).
- Overall Survival: At the second interim analysis (median follow-up of 25.7 months), the median OS was 21.4 months for belzutifan and 18.1 months for everolimus. At 18 months, the survival rates were 55.2% and 50.6%, respectively. The hazard ratio for death was 0.88 (P=0.20), which did not meet the prespecified significance criterion.
Safety Profile:
Grade 3 or higher adverse events were reported in 61.8% of the belzutifan group and 62.5% of the everolimus group. Treatment discontinuation due to adverse events occurred in 5.9% of participants receiving belzutifan, compared to 14.7% of those on everolimus.
Everolimus, which up to now was assumed as the last active option in heavily pretreated, advanced ccRCC patients, had been approved based on a placebo-controlled, phase III (RECORD-1) study, which, beyond the significant improvement of PFS with everolimus failed to demonstrate any benefit in terms of OS or ORR with the use of the mTOR inhibitor. In the LITESPARK-005 study, belzutifan significantly improved ORR and PFS, and, taking into account the gradual flattening of the PFS curve, one may still need time to validate the impact of belzutifan on OS.”
Source: Piotr Wysocki/LinkedIn
About Piotr Wysocki
Piotr Wysocki leads the Clinical Oncology Department at University Hospital and the Faculty of Oncology at Jagiellonian University-Medical College in Krakow, Poland. As an advisor to the Polish Ministry of Health, he shapes the national cancer strategy.
His clinical expertise spans the systemic treatment of breast, gynecologic, and genitourinary cancers, with a focus on developing innovative metronomic chemotherapy-based therapies for advanced cancer patients who have undergone prior treatment.
Read other posts by Piotr Wysocki published on OncoDaily.


