In the rapidly evolving field of oncology, we are witnessing remarkable advancements in various treatment modalities that promise to reshape cancer care.
Our editorial team has identified 10 cancer drugs that, while not yet FDA-approved, have garnered significant attention due to their impressive preliminary data and potential to offer innovative solutions for a range of malignancies.
This article explores Glecirasib, one of the drugs featured in OncoDaily’s “10 Most Promising Cancer Drugs Not Yet Approved: Solid Tumors – 2024 edition,” showcasing its potential to revolutionize treatment for KRAS G12C mutant cancers.
About Jacobio Pharmaceuticals
Jacobio Pharmaceuticals is a clinical-stage biopharmaceutical company specializing in developing small molecules towards “undruggable” targets in oncogenic pathways for cancer treatment. Founded in 2015 and headquartered in Beijing, China, Jacobio has built a robust pipeline of novel small molecules targeting critical signaling pathways in cancer, including KRAS, p53, myc, and immune-oncology. The company’s lead candidate, glecirasib, a KRAS G12C inhibitor undergoing an NDA review at the China CDE, exemplifies its commitment to addressing unmet medical needs through cutting-edge science and drug development. With a global presence and collaborations with leading research institutions, Jacobio is poised to make significant contributions to the field of targeted cancer therapies.
About Glecirasib
Glecirasib (JAB-21822) is an investigational small molecule inhibitor of KRAS G12C mutations being developed by Jacobio Pharmaceuticals for the treatment of various solid tumors. As a highly selective, covalent KRAS G12C inhibitor, glecirasib represents a promising new approach to targeting one of the most common and challenging oncogenic drivers in cancer.
Mechanism of Action
KRAS is a small GTPase that acts as a molecular switch, cycling between an inactive GDP-bound state and an active GTP-bound state to regulate cell proliferation and survival pathways. Oncogenic KRAS mutations, particularly at codon 12, impair GTP hydrolysis and lock KRAS in its active, GTP-bound conformation, leading to constitutive activation of downstream signaling cascades that drive tumorigenesis.
The G12C mutation, which substitutes the cysteine for glycine at position 12, occurs in approximately 13% of non-small cell lung cancers (NSCLC), 3-5% of colorectal cancers, and 1-2% of pancreatic cancers. KRAS was deemed undruggable until the recent discovery of a switch-II pocket by Dr. Shokat and his colleagues using crystallography. This G12C mutation creates a unique allosteric regulatory site for KRAS G12C specific inhibitors.
Glecirasib is designed to selectively and irreversibly bind to the cysteine residue of KRAS G12C in its inactive GDP-bound state. By covalently modifying the mutant protein, glecirasib locks KRAS G12C in the inactive conformation, preventing its activation and blocking downstream oncogenic signaling. This mechanism effectively “turns off” the hyperactive KRAS G12C, leading to the inhibition of tumor cell proliferation and induction of apoptosis.
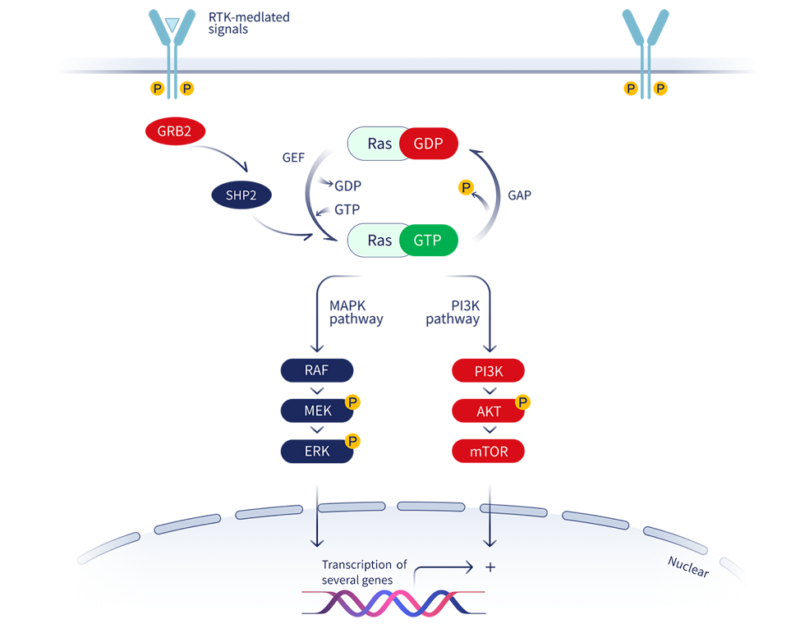
Key features of Glecirasib’s mechanism include:
- High selectivity for the G12C mutant form of KRAS over wild-type KRAS, HRAS, or NRAS
- Irreversible covalent binding to Cys12, providing durable target engagement
- Binding to the GDP-bound state, trapping KRAS G12C in its inactive conformation
- Potent inhibition of MAPK pathway signaling and other KRAS-downstream oncogenic pathways
Preclinical Data
In preclinical studies, glecirasib demonstrated potent anti-tumor activity in cell lines and xenograft models harboring KRAS G12C mutation. Key findings from these studies include:
- Subnanomolar IC50 activities against KRAS G12C mutant cell lines across multiple tumor types
- Dose-dependent inhibition of ERK phosphorylation and other KRAS downstream effectors
- Significant tumor growth inhibition and regression in NSCLC, colorectal cancer and pancreatic cancer xenograft models
- Favorable pharmacokinetic properties, including high oral bioavailability
- Minimal activity against wild-type KRAS, HRAS or NRAS, supporting a wide therapeutic window
Clinical Development and Results
Dose-escalation and expansion studies
First-in-human studies of glecirasib consist of dose-escalation and expansion trials (NCT05009329, NCT05002270) in patients with advanced solid tumors harboring KRAS G12C mutation. The studies enrolled 224 patients worldwide into different dosing regiments with a total daily dose of up to 1200 mg, including 200-800 mg once daily, 400 mg twice daily and 400 mg three times a day.
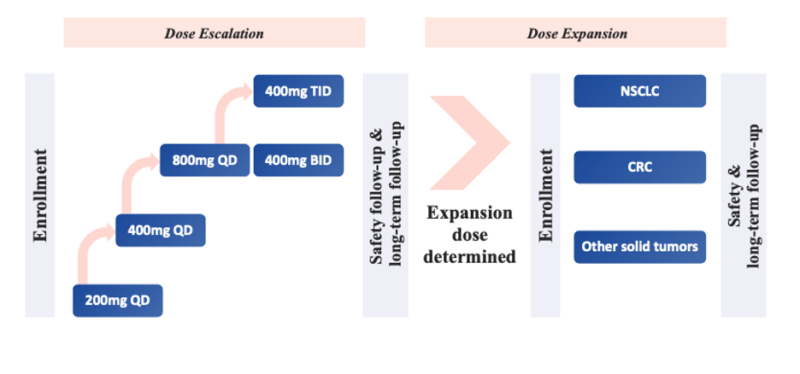
Key findings from this study included:
- Well-tolerated safety profile with no dose-limiting toxicities observed
- Maximum tolerated dose not reached; 800 mg once daily selected as recommended phase 2 dose
- Promising early efficacy signals, with objective responses observed across multiple tumor types
Glecirasib monotherapy in patients with previously treated advanced NSCLC
Based on the encouraging phase 1/2 results, glecirasib advanced to phase 2 pivotal studies in multiple indications. The pivotal phase 2b study in NSCLC (NCT05009329) enrolled 119 patients with KRAS G12C mutant advanced NSCLC who had progressed on prior platinum-based chemotherapy and immunotherapy. Patients received glecirasib 800 mg once daily until disease progression or unacceptable toxicity.
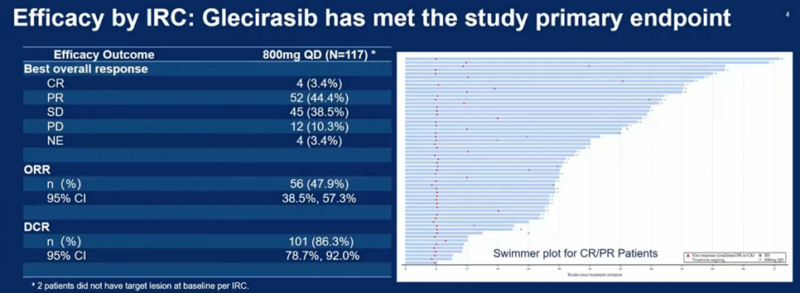
The trial met its primary endpoint, demonstrating an objective response rate (ORR) of 47.9% (95% CI: 38.5-57.3%) by independent review.
Key efficacy results included:
- Median progression-free survival (PFS) per IRC was 8.2 months (95% CI: 5.5-13.1 months)
- Median overall survival (OS) of 13.6 months (95% CI: 10.9 months – NE)
- Disease control rate of 86.3% (95% CI: 78.7-92.0%)
- The majority of CR/PR patients are ongoing. The median duration of response has not been reached; 6-month and 12-month rDoR rates were 73.6% and 56.6%, respectively
Glecirasib also demonstrated a favorable safety and tolerability profile in this study:
- Treatment-related adverse events (TRAEs) occurred in 97.5% of patients; grade ≥3 TRAEs in 38.7% No grade 5 (fatal) TRAE occurred.
- Most common TRAEs included anemia, blood bilirubin increased, and alanine aminotransferase increased/ aspartate aminotransferase increased.
- Low rates of gastrointestinal toxicities compared to other KRAS G12C inhibitors (nausea 7%, vomiting 7.6%, diarrhea 3.4%)
- Treatment discontinuation due to TRAEs occurred in 5.0% of patients
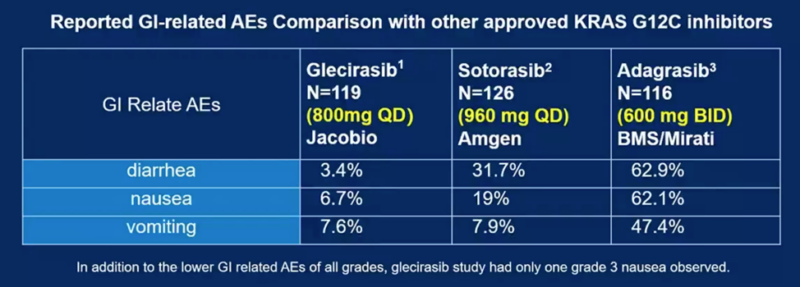
Glecirasib demonstrated favorable efficacy and tolerability in previously treated NSCLC patients, compared with FDA-approved KRAS G12C inhibitors sotorasib and adagrasib. The low incidence of gastrointestinal side effects will help to improve the patient’s quality of life and treatment adherence. These results suggestglecirasib as an alternative treatment for patients with previously treated advanced NSCLC.
Glecirasib monotherapy in patients with previously treated advanced pancreatic cancer or other solid tumors
In 48 evaluable patients, glecirasib demonstrated a confirmed ORR of 50% and a disease control rate of 93.8%. In the pancreatic cancer cohort specifically (n=31), the confirmed ORR was 41.9% with a median PFS of 5.6 months and median OS of 10.7 months. These results are particularly encouraging given the historically poor outcomes in pancreatic cancer and the limited efficacy of other KRAS G12C inhibitors in this indication.
Glecirasib Ongoing Clinical Trials
Several additional clinical trials of glecirasib are currently ongoing or planned:
- Phase 3 registration study of glecirasib plus the SHP2 inhibitor JAB-3312 versus tislelizumab combined with pemetrexed and carboplatin in the frontline treatment of advanced KRAS G12C mutant NSCLC (NCT06416410)
- Phase 2 single-arm pivotal study of glecirasib monotherapy in KRAS G12C mutated pancreatic cancer(NCT06008288)
- Phase 2 study of glecirasib in combination with cetuximab in KRAS G12C mutant colorectal cancer (NCT05194995)
- Phase 2 single-arm pivotal study of glecirasib monotherapy in KRAS G12C mutated pan-tumor basket patients (biliary tract cancer, gastric cancer, small bowel cancer, etc.)
These studies aim to further evaluate glecirasib’s efficacy as monotherapy and in rational combinations designed to enhance activity and overcome potential resistance mechanisms.
Unique Features and Potential Advantages
Several characteristics of glecirasib suggest it may have advantages over other KRAS G12C inhibitors in development:
- High selectivity for KRAS G12C over wildtype KRAS, HRAS, or NRAS, supporting a wild therapeutic window and a favorable safety profile.
- Encouraging clinical outcomes across various tumor types with KRAS G12C mutation, including NSCLC, pancreatic cancer, colorectal cancer, and other solid tumors.
- Low gastrointestinal toxicity in patients: the safety profile of glecirasib, particularly the low rates of nausea, vomiting, and diarrhea, may allow for improved tolerability and treatment adherence.
- Potential for combination strategies: synergistic activity when glecirasib is combined with other targeted therapies, such as SHP2 inhibitors or EGFR inhibitors.
Development Status and Future Directions
Glecirasib is currently in late-stage clinical development, with pivotal trials completed or ongoing in multiple indications. Based on the positive results in patients with previously treated advanced NSCLC, Jacobio Pharmaceuticals submitted a new drug application to China’s National Medical Products Administration in May 2024 and glecirasib was granted Priority Review.
The U.S. Food and Drug Administration (FDA) has granted glecirasib Orphan Drug Designation for pancreatic cancer, recognizing its potential to address an area of high unmet need. Additionally, glecirasib received Breakthrough Therapy Designation from China’s Center for Drug Evaluation for second-line and later treatment of KRAS G12C mutant NSCLC and pancreatic cancer.
Future development plans for glecirasib include:
- Expansion into earlier lines of therapy, including first-line settings in combination with other agents
- Evaluation in additional tumor types harboring KRAS G12C mutations
- Development of combination strategies to enhance efficacy and overcome resistance mechanisms
Conclusion
Glecirasib represents a promising new approach to targeting KRAS G12C mutation, a long-sought therapeutic goal in oncology. With its unique chemical structure, potent and selective mechanism of action, and encouraging clinical data, glecirasib has the potential to become a best-in-class KRAS G12C inhibitor. The ongoing clinical development program will further elucidate its efficacy and safety across multiple tumor types and treatment settings.
As our understanding of KRAS biology and resistance mechanisms continues to evolve, glecirasib and other targeted therapies offer hope for improved outcomes in patients with KRAS-driven cancers. The coming years will be critical in determining the ultimate place of glecirasib in the therapeutic landscape and its potential to transform the treatment paradigm for patients with KRAS G12C mutant tumors.
