Amol Akhade, Senior Medical and Hemato-Oncologist based in Mumbai, India, shared a post on LinkedIn:
“First-line treatment options for NSCLC with uncommon EGFR mutations: a rapidly evolving landscape with growing prospective data.
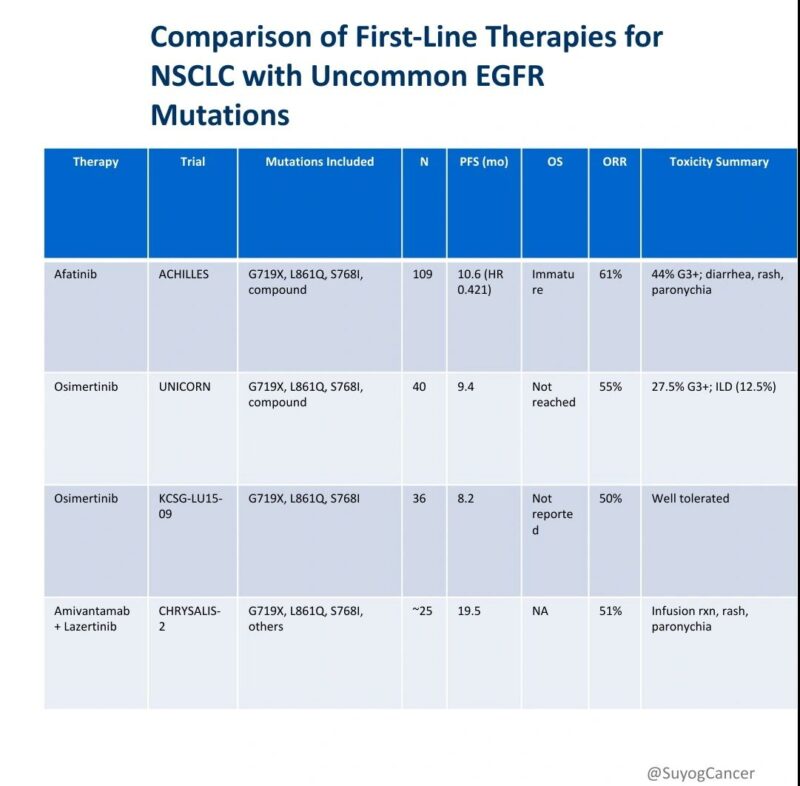
Historically, therapeutic strategies for patients harboring uncommon EGFR mutations (e.g., G719X, L861Q, S768I, and compound variants) have been guided by retrospective analyses or extrapolated from data in common EGFR mutations. Recent prospective studies have begun to define evidence-based standards specific to this biologically heterogeneous population.
This slide summarizes available prospective first-line data:
1. Afatinib – ACHILLES/TORG1834
Phase III randomized trial (afatinib vs platinum-pemetrexed) N = 109 Median PFS: 10.6 months HR: 0.421 (95% CI: 0.251–0.706; P=0.0010) ORR: 61.4% Toxicity: Predominantly dermatologic/GI; 44% grade ≥3 Notable efficacy in compound mutations (PFS ~21 months)
2. Osimertinib – UNICORN
Phase II, single-arm trial N = 40 Median PFS: 9.4 months ORR: 55% Toxicity: Grade ≥3 AEs in 27.5%; ILD in 12.5% Potentially limited efficacy in PACC-class mutations (e.g., G719X, S768I)
3. Osimertinib – KCSG-LU15-09
Phase II, single-arm study N = 36 Median PFS: 8.2 months ORR: 50% Included G719X, L861Q, and S768I Favorable safety profile
4. Amivantamab + Lazertinib – CHRYSALIS-2 (Cohort C)
Phase I/Ib expansion cohort N ≈ 25 (EGFR-TKI–naïve, uncommon EGFR mutations) Median PFS: 19.5 months (95% CI: 11.0–NE) ORR: 51% Toxicity: Infusion reactions, rash, hypoalbuminemia
Gaps in the field remain:
- No prospective head-to-head comparisons between afatinib and osimertinib
- PFS hazard ratios are underreported in single-arm trials
- CNS efficacy and mutation-structure response correlations (e.g., PACC vs classical) require further clarification
This slide is intended as a concise educational resource for oncologists, trainees, and academic centers managing patients with rare EGFR mutation subsets.
Please feel free to share or use in journal clubs, MDTs, or lecture settings.”