
Piotr Wysocki: Datopotamab deruxtecan – another anti-TROP2 conjugate demonstrating activity in advanced ER+/HER2- and triple-negative breast cancer patients
Piotr Wysocki recently shared on LinkedIn:
“Datopotamab deruxtecan – a novel anti-TROP2 conjugate demonstrates activity in advanced ER+/HER2- and triple-negative breast cancer patients.
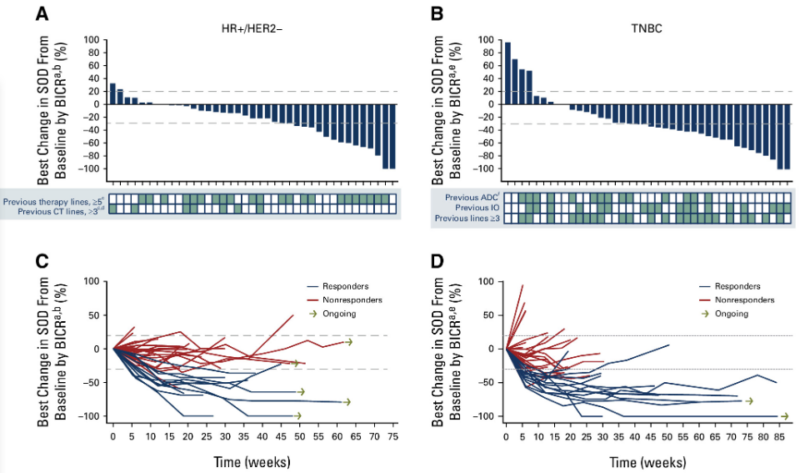
A phase I TROPION-PanTumor01 study evaluated datopotamab deruxtecan (Dato-DXd) – an anti-trophoblast cell-surface antigen 2 (TROP2) antibody-drug conjugate carrying a topoisomerase I inhibitor payload attached via a plasma-stable, selectively cleavable linker. In a paper published online in the Journal of Clinical Oncology, Bardia A, et al. presented data on Dato-DXd activity in 85 heavily pretreated patietns with advanced ER+/HER2- (n: 41) and triple-negative (n:44) breast cancer.
Dato-DXd treatment was associated with:
- ORR – 26.8% (ER+/HER2-), 31.8% (TNBC),
- DCR – 85.4% (ER+/HER2-), 79.5% (TNBC),
- Median PFS – 8.3 months (ER+/HER2-), 4.4 months (TNBC),
- Median OS – NE (ER+/HER2-), 13.5 months (TNBC),
- Grade 3-4 AE – 41.5% (ER+/HER2-), 52.3% (TNBC).”
Read further.
Source: Piotr Wysocki/LinkedIn
Piotr J. Wysocki leads the Clinical Oncology Department at University Hospital and the Faculty of Oncology at Jagiellonian University-Medical College in Krakow, Poland. He has supervised four PhDs, trained numerous oncology residents, and contributed to national guidelines for genitourinary and breast cancer treatment. As an advisor to the Polish Ministry of Health, he shapes the national cancer strategy. Wysocki established early-phase clinical study units and specializes in systemic treatment for breast, gynecologic, and genitourinary cancers.His clinical expertise spans the systemic treatment of breast, gynecologic, and genitourinary cancers, with a focus on developing innovative metronomic chemotherapy-based therapies for advanced cancer patients who have undergone prior treatment. His scientific pursuits encompass the development and utilization of Immunopheresis in solid tumor treatment and the management of immune-related adverse events. He has led over 70 international clinical studies and published extensively in cancer research. Since 2023, he has been a member of the American Society of Clinical Oncology International Affairs Committee.
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
