Observed annually on May 20, International Clinical Trials Day commemorates a pivotal moment in medical history—the 1747 scurvy trial by James Lind. Aboard the HMS Salisbury, Lind conducted one of the earliest controlled clinical trials, treating 12 sailors with various remedies. His discovery—that citrus fruits rapidly cured scurvy—was later published in the landmark Treatise of the Scurvy (1753), a work widely regarded as the foundation of modern clinical trials.
Today, this day reminds us that clinical trials remain the cornerstone of medical advancement, shaping the future of treatments, vaccines, and public health.
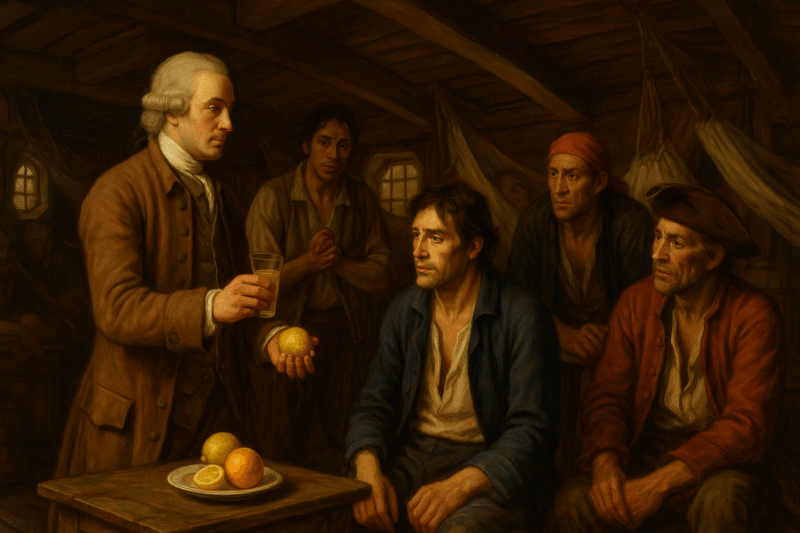
The Global Impact of Clinical Trials
Clinical trials have revolutionized healthcare, providing the evidence base for life-saving innovations:
-
HIV/AIDS therapies
-
Targeted cancer treatments
-
COVID-19 vaccines
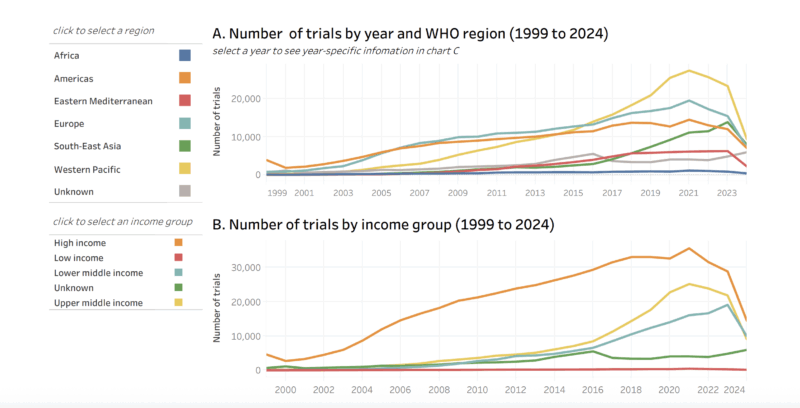
According to the World Health Organization (WHO):
-
Global trial registrations have surged, especially during the COVID-19 pandemic.
-
In 2023, the Western Pacific region (led by China and Japan) registered ~23,250 new trials, while Africa registered only 845.
-
In 2022, high-income countries conducted over 27,000 trials, compared to 24,800 in low- and middle-income countries (LMICs).
These disparities highlight both the growing scale of research and ongoing inequities in access and representation.
Yet, clinical trials are not without challenges:
-
Cost: Developing a new drug can exceed $1 billion and take over 10 years.
-
Recruitment: More than 80% of trials fail to recruit on schedule.
-
Approval odds: Only about 1 in 7 drugs entering Phase I trials eventually receive regulatory approval.
To meet these challenges, we need innovation, ethical rigor, and global cooperation.
Innovations in Trial Design and Technology
Modern clinical trials are transforming rapidly through technology:
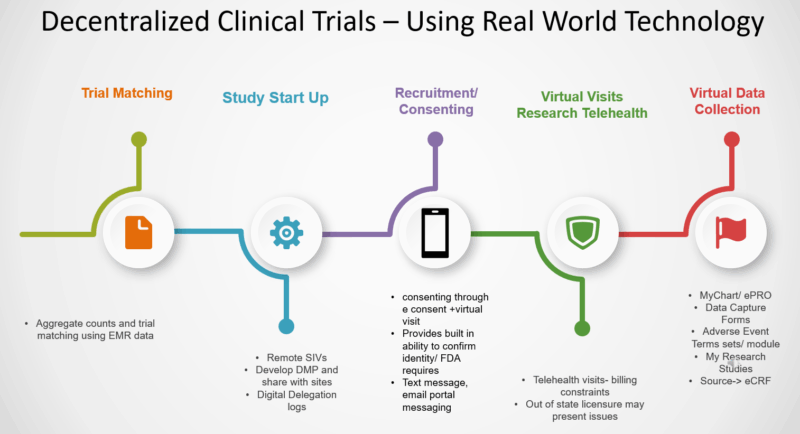
1. Decentralized Trials
-
Enabled by telemedicine, mobile health apps, and home testing
-
Reduce patient burden and increase access for rural, elderly, and minority populations
-
Improve diversity and inclusivity by overcoming geographic and socioeconomic barriers
2. Artificial Intelligence (AI) and Data Science
-
AI can optimize trial design, simulate outcomes, and enhance efficiency
-
TrialGPT, an NIH-developed AI, matched ~90% of relevant trials and cut clinician screening time by 40%
-
Generative AI can simulate data trends and draft study documents to boost trial success
3. Digital Health Tools
-
Wearables track vital signs continuously
-
Electronic consent (e-consent) tools make participation more accessible and informed
-
Interactive platforms improve engagement and data quality
Together, these technologies are making trials faster, smarter, and more patient-centered.
Upholding Ethics, Standards, and Diversity
Ethical oversight is the bedrock of trustworthy research. Key principles include:
-
Informed consent
-
Scientific soundness
-
Transparent protocols
-
Participant safety
Institutions follow international standards like the Declaration of Helsinki and ICH-GCP (Good Clinical Practice).
Diversity in Trials
Despite growing awareness, representation gaps persist:
-
Less than 5% of trials included pregnant women in 2022
-
Only 13% included children
-
LMICs and minority populations remain underrepresented
WHO now urges stakeholders to:
-
Embed research in routine care
-
Improve participant diversity through targeted outreach and inclusive trial designs
-
Use decentralized trials to boost equity
International Collaboration and Harmonization
Multinational trials are essential for globally applicable evidence:
-
Initiatives like WHO Solidarity and RECOVERY have set benchmarks for international coordination
-
Regulatory harmonization—led by bodies like the International Council for Harmonisation (ICH)—allows shared protocols and faster approvals
-
In May 2025, WHO launched a Global Action Plan promoting:
-
Strengthened research capacity in low-resource settings
-
Coordinated multicenter trials
-
Infrastructure development for equitable research access
-
Global cooperation ensures that innovations benefit all populations, not just a few.
Key Trends Shaping Clinical Trials Today
1. Adaptive and Platform Trials
-
Flexibly adjust treatment arms and sample sizes based on early data
-
Speed discovery and reduce patient exposure to ineffective treatments
2. Digital Recruitment and E-Consent
-
Use of social media, patient portals, and EHR alerts for outreach
-
Online pre-screening and telehealth visits lower barriers to entry
3. Decentralized Data Collection
-
Home-based kits, mobile apps, and wearables provide continuous monitoring
-
Support real-world data and reduce the need for in-person visits
4. AI-Driven Trial Operations
-
Predict adverse events, optimize site selection, and ensure data quality
-
Increase trial success rates through smart analytics
5. Patient-Centric Engagement
-
Include patient input in trial design
-
Share results and focus on patient-reported outcomes
-
Build trust and relevance through community participation
These trends are reshaping clinical research into a more efficient, ethical, and inclusive enterprise.
Conclusion: A Vision for the Future
From James Lind’s lemon cure to today’s AI-powered global trials, clinical research continues to drive medical progress. On International Clinical Trials Day, we honor the legacy of pioneers while embracing the innovations of tomorrow.

The path forward demands:
-
Embracing digital and decentralized technologies
-
Upholding ethical standards and diversity
-
Fostering global partnerships
As WHO emphasizes, building robust clinical trial ecosystems will ensure equitable access to research and generate high-quality evidence that truly serves local populations.
In 2025 and beyond, let us commit to clinical research that is inclusive, innovative, and globally impactful—because every trial is a step toward better health for all.
Let’s Keep the Momentum Going
As we honor the past and shape the future of clinical research, each of us—whether patients, professionals, or advocates—has a role to play. Stay informed, stay engaged, and support efforts that make trials more inclusive and impactful.
Follow OncoDaily for more insights, stories, and breakthroughs from the world of clinical research and oncology. Together, we’re building a healthier tomorrow.
Written by Md Foorquan Hashmi, MD, Sr. Editor, OncoDaily: India Bureau


