Al-Ola A Abdallah, Associate Professor, Plasma Cell Disorder Program Director, Division of HMCT/University of Kansas Medical Center, posted the following on X:
“Idecabtagene vicleucel or ciltacabtagene autoleucel for RRMM!
This study aims to directly compare the efficacy and safety of ide-cel and cilta-cel in a real-world, through a retrospective, observational, multi-center study including patients from several international centers (primarily in Germany, Switzerland, and Spain)

Pt Characterstics:
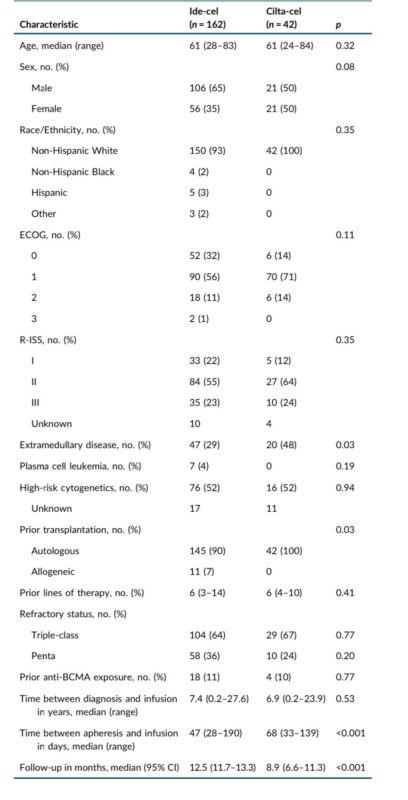
- A total of 204 patients were included: 162 received ide-cel, and 42 received cilta-cel.
- Median age 61 years for both groups
- Turnaround time between apheresis and infusion was significantly longer for cilta-cel (68 days vs. 47 days for ide-cel; p<0.001)
- Most patients had an ECOG performance status of 0 or 1 at time of infusion.
- A notable difference was that a higher percentage of cilta-cel patients had EMD (48% vs 29% in the ide-cel group, p=0.03).
- Most patients were triple-class refractory.
Efficacy:
- ORR: Cilta-cel showed a significantly higher ORR (93%) than ide-cel (79%; p<0.001).
- Complete Response: Cilta-cel had a higher complete response rate at day 30 (48% vs. 26% for ide-cel; p<0.001)
- PFS: 10-month PFS was significantly higher with cilta-cel (82%) compared to ide-cel (47%; p<0.001)
- OS: While not statistically significant (p=0.06), there was a trend towards improved 10-month OS for cilta-cel (90%) versus ide-cel (77%)
Safety:
- CRS: Similar overall incidence of CRS (81% for cilta-cel vs. 85% for ide-cel; p=0.51)
- Severe CRS (Grade 3-4) was more frequent in the cilta-cel group (10% vs 4%).
- CRS onset was significantly earlier for ide-cel (median of 2 days) compared to cilta-cel (median of 4 days; p < 0.001).
ICANS: Similar overall incidence of ICANS (19% for both)
- Severe ICANS (Grade 3-4) was more frequent in the cilta-cel group (7% vs 2%).
- ICANS onset was also earlier for ide-cel (median of 3 days) vs cilta-cel (median of 6 days; p<0.001).
- Delayed Neurotoxicity: Observed in some patients in both groups, which was resolved with steroids
NRM: Similar between groups, 5% for cilta-cel vs. 3% for ide-cel (p=0.51) and lower than seen in a recent meta-analysis.
- Causes of Death: Relapse was a more frequent cause of death in the ide-cel group, while infection/septic shock was more common in the cilta-cel group. One case of Hemophagocytic lymphohistiocytosis (HLH) related death occurred in the cilta-cel group.
- Resource Utilization: The median hospital stay was longer for cilta-cel (17 days vs 14 days, p=0.002).
- Corticosteroid use was more frequent in the cilta-cel group (35% vs 26%, p=0.04)
Ide-cel expansion peaked earlier (Day 7), whereas cilta-cel expansion peaked later (Day 14).
- Cilta-cel expansion was associated with ICANS, with higher CAR-T levels in patients who developed ICANS.
- Cilta-cel showed improved outcomes across most clinical subgroups (age, sex, risk factors etc.)
The MyCARe model was prognostic for PFS in both treatment groups and cilta-cel showed significantly improved outcomes for each risk group
Real-World vs Clinical Trial Data: The results highlight potential differences between real-world outcomes and those observed in clinical trials, particularly regarding NRM, and highlight the importance of real-world data. The authors speculate that the experience gained in centers is likely improving their management of toxicities like CRS/ICANS.
Limitations
- Retrospective design.
- Smaller sample size for the cilta-cel group.
- The selection of treatment was as per center’s discretion, potentially leading to bias.
- Longer follow-up for ide-cel group.
- The study only included patients with triple-class exposed RRMM, which limits the application to earlier lines of therapy.
- Limited number of non-white individuals included in the study, limiting the value to study ethnic disparities.
This real-world, multi-center study demonstrates that cilta-cel is associated with superior outcomes compared to ide-cel in triple-class exposed RRMM, despite having distinct cellular dynamics and toxicity profiles.”


