Vivek Subbiah shared a post on X/Twitter:
“Hot off the press presenting to the Nature Portfolio precision oncology. Darwinian off-target resistance mechanisms to selective RET inhibition in RET driven cancer. Acquired NTRK3 fusion, ALK fusion, NTRK3 solvent-front mutation as secondary, tertiary, and quaternary off-target resistance mechanisms.
Patients treated with RET protein tyrosine kinase inhibitors (TKIs) selpercatinib or pralsetinib develop RET TKI resistance by secondary RET mutations or alterative oncogenes.
Alterative oncogenes pose a greater challenge for disease management because of multiple potential mechanisms and the unclear tolerability of drug combinations.
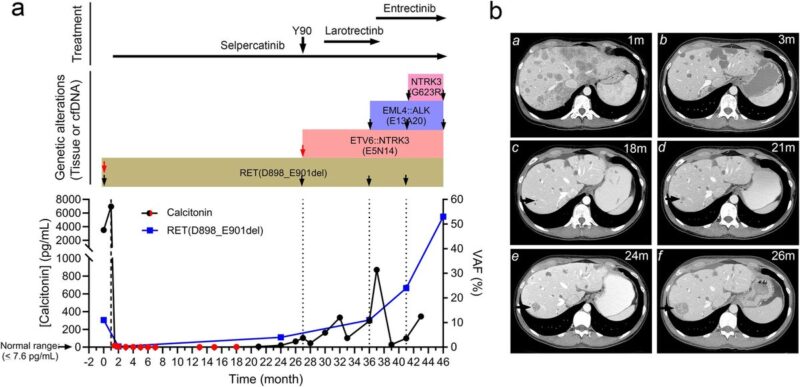
Thyroid carcinoma harboring a RET activation loop D898_E901del mutation treated with selpercatinib. Molecular alterations were monitored with tissue biopsies and cfDNA during the treatment.
The selpercatinib-responsive MTC progressed with an acquired ETV6::NTRK3 fusion, which was controlled by selpercatinib plus the NTRK inhibitor larotrectinib.
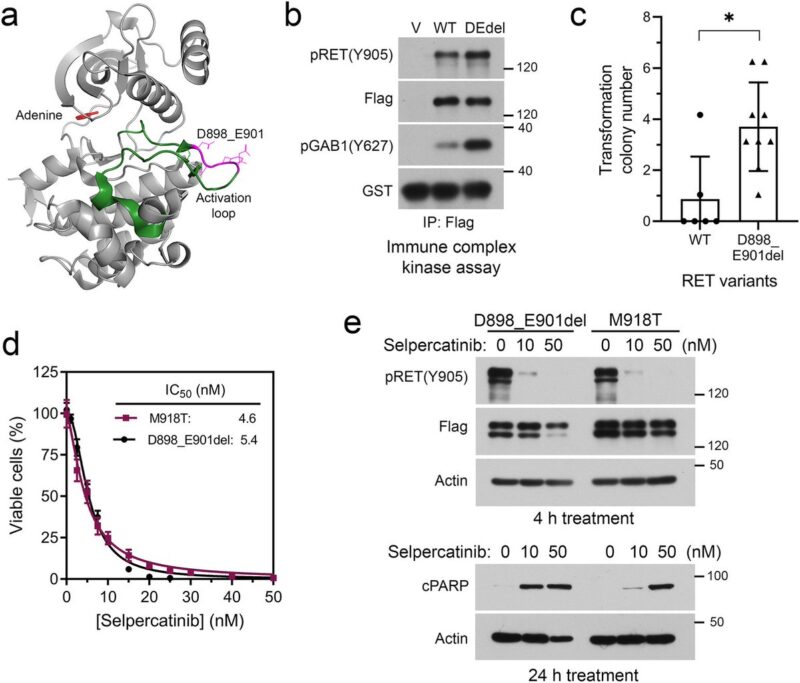
Subsequently, tumor progressed with an acquired EML4::ALK fusion. Combination of selpercatinib with the dual NTRK+ALK inhibitor entrectinib reduced the tumor burden, which was followed by appearance of NTRK3 solvent-front G623R mutation.
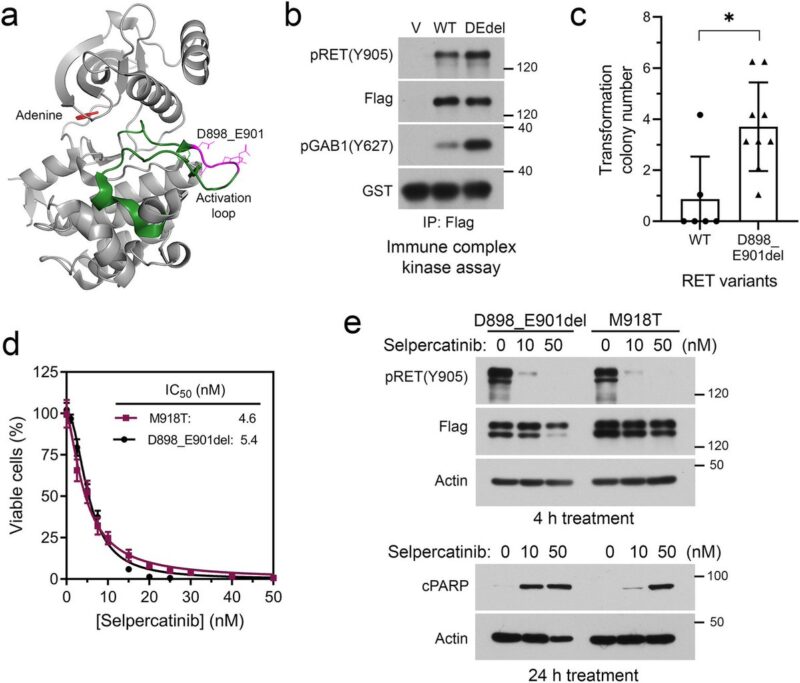
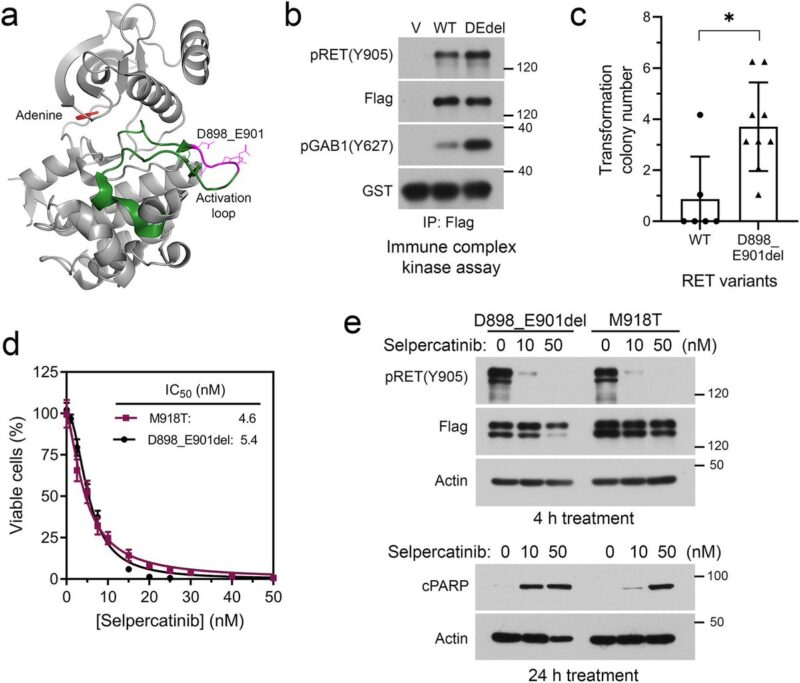
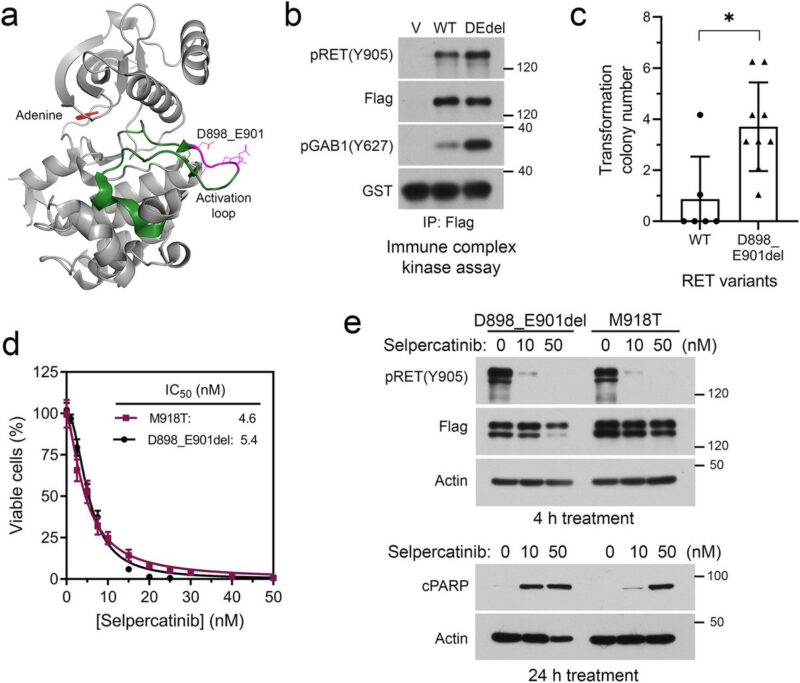
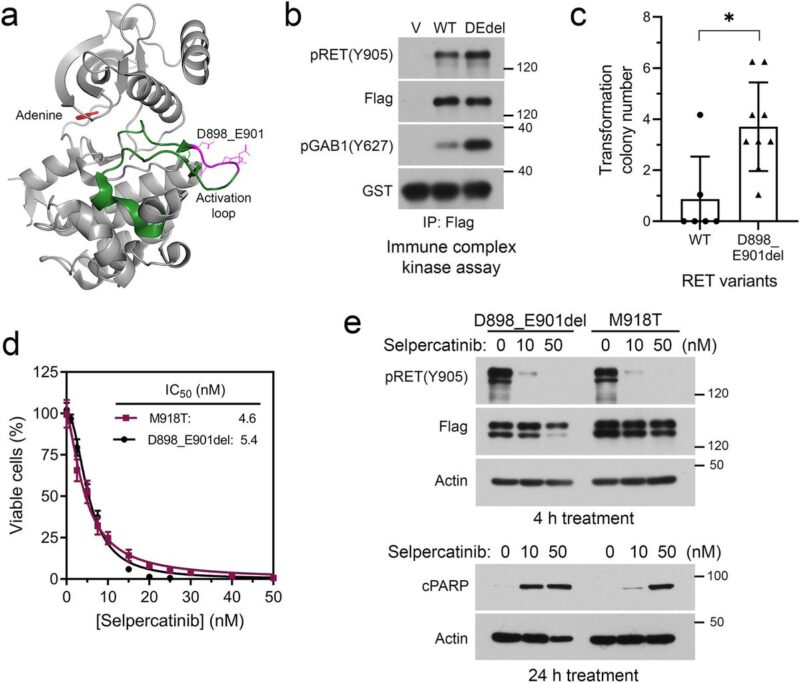
Preclinical experiments validated selpercatinib plus larotrectinib or entrectinib inhibited RET/NTRK3 dependent cells, whereas selpercatinib plus entrectinib was necessary to inhibit cells with RET/NTRK3/ALK triple alterations or a mixture of cell population carrying these genetic alterations.
Thus, RET-altered MTC adapted to selpercatinib and larotrectinib with acquisition of ETV6::NTRK3 and EML4::ALK oncogenes can be managed by combination of selpercatinib and entrectinib providing proof-of-concept of urgency of incorporating molecular profiling in real-time and personalized N-of-1 care transcending one-size-fits-all approach.
This case demonstrates the complexity of cancer evolution in RET-targeted therapy and the possibility of addressing such complexity using customized combination therapies that prolong tumor control. It also illustrates the utility of using liquid biopsy to guide timely decisions of personalized treatments (i.e. n-of-1 trials).
Patient-driven discovery and post-clinical validation of NTRK3 fusion as an acquired resistance mechanism to selpercatinib in RET fusion-positive lung cancer.
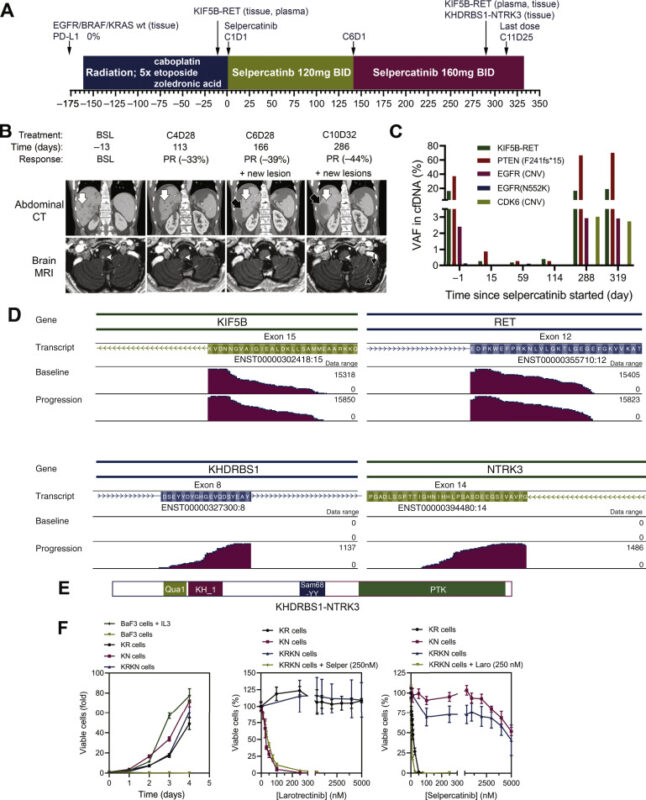
Structural basis of acquired resistance to selpercatinib and pralsetinib mediated by non-gatekeeper RET mutations – Annals of Oncology.
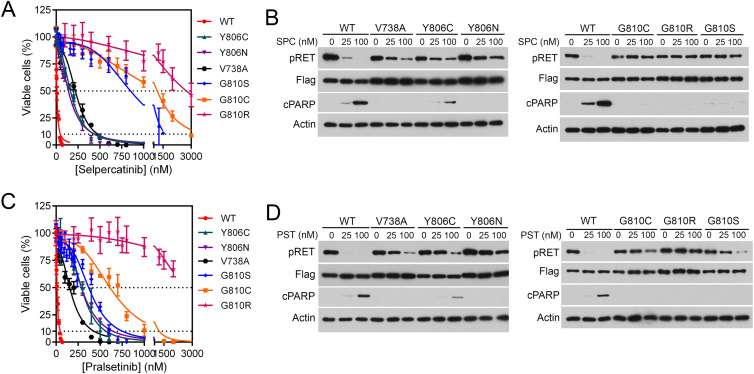
Selective RET kinase inhibition for patients with RET-altered cancers – Annals of Oncology.”
Source: Vivek Subbiah/X
Vivek Subbiah is the Chief of Early-Phase Drug Development at the Sarah Cannon Research Institute (USA). He is the former Executive Director of Oncology Research at the MD Anderson Cancer Network and a former Associate Professor in the Department of Investigational Cancer Therapeutics at The University of Texas MD Anderson Cancer Center.
Dr. Vivek Subbiah has served as the principal investigator in over 100 phase I/II trials and co-investigator in over 200 clinical trials and is known for his leadership in several first-in-human and practice-changing studies that directly led to approvals from the FDA, European Medicines Agency, and other agencies across the world. He is an expert in tumor agnostic precision oncology and leads the BRAF and RET tissue agnostic studies to FDA approval.


