Vivek Subbiah shared on X/Twitter:
“By popular demand delighted to share the ‘Top Precision Oncology articles of 2023’.
Remember, this list isn’t exhaustive!
Feel free to Tag, add, tweet, X and share your must-reads with this list. Let’s keep the knowledge flowing!
‘The menin inhibitor revumenib in KMT2A-rearranged or NPM1-mutant leukaemia’ nature.
The interaction of menin with lysine methyltransferase 2A (KMT2A), an epigenetic regulator, is a dependence in acute leukaemia caused by either rearrangement of KMT2A or mutation of the nucleophosmin 1 gene (NPM1). This phase 1 study showed that targeting critical epigenetic regulators reverses aberrant transcription in cancer, thereby restoring normal tissue function.
Added this study as it is a prime example of thinking about patients when designing trials – ‘a patient-centric clinical trial’ Mark Lewis Tatiana Prowell Mel Mann
A phase 2 adaptive umbrella trial consisting of a criteria-fulfilled (CF) cohort and a compassionate use (CU) cohort under expanded eligibility criteria, and a prospective real-world study (RWS). First-line pyrotinib in advanced HER2-mutant non-small-cell lung cancer: a patient-centric phase 2 trial.
Two BRAF precision medicine practice changing studies were published #1 BRAF practice changing study
Dabrafenib plus trametinib in BRAFV600E-mutated rare cancers: the phase 2 ROAR trial
ROAR basket trial + NCI match + Pediatric trial – pooled analysis led to FDA approval of Dabrafenib plus trametinib in BRAFV600 positive cancers. A huge milestone in Tissue Agnostic Precision Medicine – Huge honor of a lifetime to see this approval through for patient access. Many folks to thank for this.
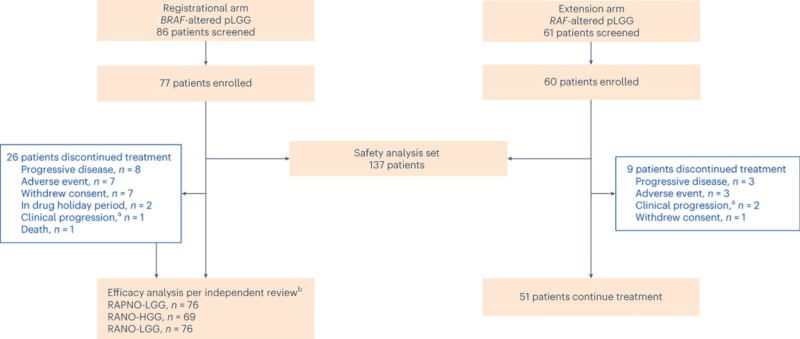
#2 BRAF practice changing study- Dabrafenib plus Trametinib in Pediatric Glioma with BRAF V600 Mutations | NEJM
Among pediatric patients with low-grade glioma with BRAF V600 mutations, dabrafenib plus trametinib resulted in significantly more responses, longer progression-free survival, and a better safety profile VS standard chemotherapy as First-line therapy
Visit the article website.
A new kid on the block -> The type II RAF inhibitor tovorafenib in relapsed/refractory pediatric low-grade glioma: the phase 2 FIREFLY-1 trial
Visit the article website.
BRAF–MEK Inhibition in Newly Diagnosed Papillary Craniopharyngiomas
Showing 94 % response rate when patients are treated upfront BRAF Precision Medicine
Visit the article website.
Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial Zolbetuximab + CAPOX represents a new first-line therapy for patients with CLDN18.2-positive, HER2-negative, locally advanced unresectable or mG/GEJ adenocarcinoma.
Visit the article website.
RLY-4008, the First Highly Selective FGFR2 Inhibitor with Activity across FGFR2 Alterations and Resistance Mutations Cancer Discovery Elizabeth McKenna AACR
In knew this drug was super potent right from the 1st patient 1st day ! Super honored to share this publication with ASCO ASCO23 meeting Patients with FGFR2-driven cancers derive limited benefit from pan-FGFRi due to multiple FGFR1–4-mediated toxicities and acquired FGFR2 resistance mutations. RLY-4008 is a highly selective FGFR2 inhibitor that targets primary alterations and resistance mutations and induces tumor regression while sparing other FGFRs, suggesting it may have broad therapeutic potential. Was published with an editorial by the amazing Sumanta K. Pal Check out the cool video in the supplemental section of FGFR1 vs FGFR2 receptor protein motion dynamics.
Visit the article website.
Editorial in Cancer Discovery by Sumanta K. Pal –> FGFR Inhibition: Understanding and Overcoming Resistance.
Visit the article website.
Adding our recent editorial Annals of Oncology Guru P. Sonpavde Unlocking precision oncology with FGFR inhibition in urothelial carcinoma – Annals of Oncology
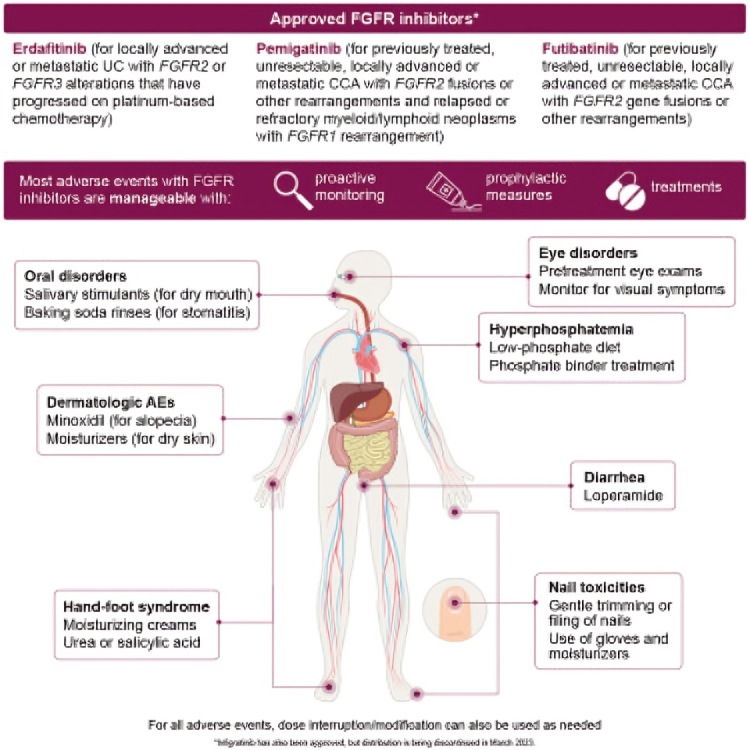
One of the challenges of FGFR inhibitors is adverse events -sharing our paper –> Clinical development and management of adverse events associated with FGFR inhibitors: Cell Reports Medicine Cell Reports Medicine
Visit the article website.
By the amazing Neeraj Agarwal First-line talazoparib with enzalutamide in HRR-deficient metastatic castration-resistant prostate cancer: the phase 3 TALAPRO-2 trial Combining talazoparib with enzalutamide significantly improved radiographic progression-free survival in patients with mCRPC harboring HRR gene alterations, supporting talazoparib plus enzalutamide as a potential first-line treatment for these patients. Nature Medicine
A H3K27M-targeted vaccine in adults with diffuse midline glioma
Visit the article website.
NVL-520 Is a Selective, TRK-Sparing, and Brain-Penetrant Inhibitor of ROS1 Fusions and Secondary Resistance Mutations Cancer Discovery Elizabeth McKenna Alexander Drilon
The combined preclinical features of NVL-520 that include potent targeting of ROS1 and diverse ROS1 resistance mutations, high selectivity for ROS1 G2032R over TRK, and brain penetration mark the development of a distinct ROS1 TKI with the potential to surpass the limitations of earlier-generation TKIs for ROS1 fusion–positive patients.
Visit the article website.
Base-Edited CAR7 T Cells for Relapsed T-Cell Acute Lymphoblastic Leukemia NEJM
CNS Efficacy of Osimertinib With or Without Chemotherapy in Epidermal Growth Factor Receptor–Mutated Advanced Non–Small-Cell Lung Cancer Journal of Clinical Oncology
Amivantamab plus Chemotherapy in NSCLC with EGFR Exon 20 Insertions | NEJM
Sotorasib plus Panitumumab in Refractory Colorectal Cancer with Mutated KRAS G12C | NEJM
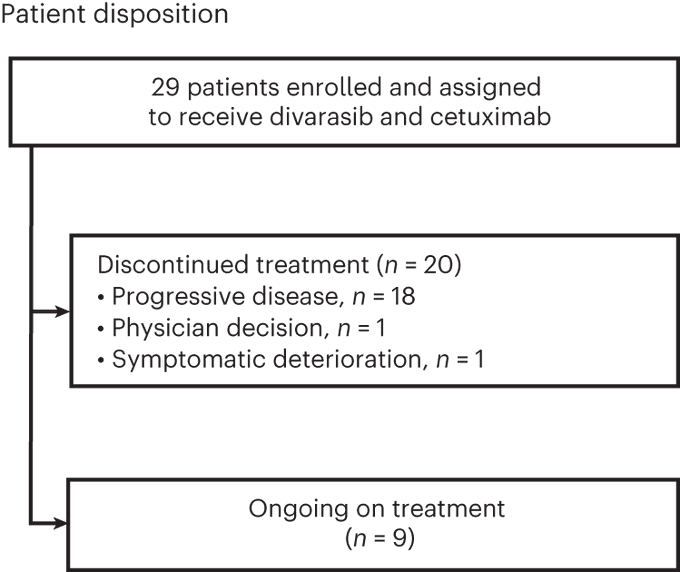
Divarasib plus cetuximab in KRAS G12C-positive colorectal cancer: a phase 1b trial
Nature Medicine
Vorasidenib in IDH1- or IDH2-Mutant Low-Grade Glioma | NEJM
The advent of selective RET inhibitors have transformed the landscape of RET altered cancers. RCT in a rare oncogenic driver established selpercatinib as standard of care in 1st line RET + NSCLC globally. First-Line Selpercatinib or Chemotherapy and Pembrolizumab in RET Fusion–Positive NSCLC | NEJM
The original study published NEJM was alone sufficient for the initial approval Efficacy of Selpercatinib in RET Fusion–Positive Non–Small-Cell Lung Cancer | NEJM
Selective RET inhibitor is now standard of care for RET + MTC Phase 3 Trial of Selpercatinib in Advanced RET-Mutant Medullary Thyroid Cancer | NEJM
Knew this drug would graduate right from the 1st patient enrolled -Selective RET kinase inhibition for patients with RET-altered cancers – Annals of Oncology.”
Source: Vivek Subbiah/X
Vivek Subbiah is the Chief of Early-Phase Drug Development at the Sarah Cannon Research Institute (USA). He is the former Executive Director of Oncology Research at the MD Anderson Cancer Network and a former Associate Professor in the Department of Investigational Cancer Therapeutics at The University of Texas MD Anderson Cancer Center. Dr. Vivek Subbiah has served as the principal investigator in over 100 phase I/II trials and co-investigator in over 200 clinical trials and is known for his leadership in several first-in-human and practice-changing studies that directly led to approvals from the FDA, European Medicines Agency, and other agencies across the world. He is an expert in tumor agnostic precision oncology and leads the BRAF and RET tissue agnostic studies to FDA approval.
