
Daniel Neumeier: FDA/CDER novel drug approvals 2023
Daniel Neumeier, Life Sciences Specialist at
“FDA/CDER novel drug approvals 2023
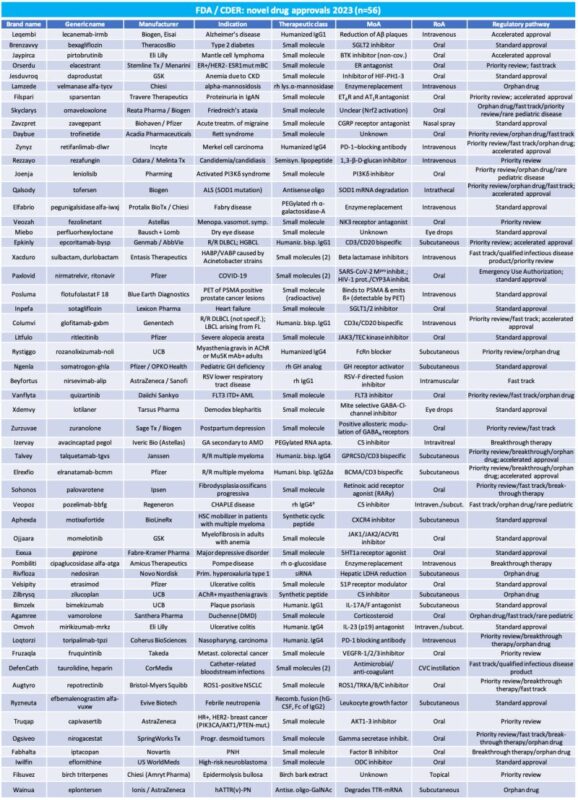
The below table lists all novel drugs which have been newly approved in 2023 by CDER as of December 26.
Key takeaways:
Number of novel drug approvals: 56 (c.51% increase compared to 2022)
Companies with highest number of approvals
• Pfizer: 6 approved drugs/11% (migraine, Covid-19, alopecia areata, pediatric GH deficiency, R/R multiple myeloma, ulcerative colitis)
• Biogen: 4/7% (Alzheimer’s, Friedreich’s ataxia, ALS, postpartum depression)
• AstraZeneca: 3/5% (RSV, breast cancer, polyneuropathy of hereditary transthyretin-mediated amyloidosis)
• Chiesi: 3/5% (alpha-mannosidosis, Fabry disease, epidermolysis bullosa)
• UCB: 3/5% (2x myasthenia gravis, plaque psoriasis)
Approvals by therapeutic class:
• Small molecules: 31/55% (includes one radioactive diagnostic agent)
• Therapeutic mAbs: 12/22% (5 IgG1, 1 IgG2, 6 IgG4)
• Peptides and proteins: 3 (semi-)synthetic (lipo-)peptides, 4 recombinant enzymes/hormones (1 PEGylated for plasma half-life extension), 1 fusion-protein (fusion to IgGFc for plasma half-life extension); (14%)
• DNA/RNA based drugs: 4/7% (1 PEGylated for plasma half-life extension)
• Other: 1 natural extract (2%)
Approvals by popular TAs
• Oncology: 17/31% (mantle cell lymphoma, ER+/HER2- ESR1 mut. mBC, merkel cell carcinoma, 2x R/R DLBCL, HGBCL, AML, 2x R/R multiple myeloma, HSC mobilizer for patients with multiple myeloma, myelofibrosis, nasopharyngeal carcinoma, metastat. Colorectal cancer, ROS1+ NSCLC, febrile neutropenia, HR+ HER2- BC, desmoid tumors, neuroblastoma)
• Neurology: 6/11% (Alzheimer’s, Friedreich’s ataxia, migraine, Rett syndrome, ALS, hATTR(v)/PN)
• Autoimmune disorders: 6/11% (Alopecia areata, 2x myasthenia gravis, 2x ulcerative colitis, plaque psoriasis)
• Infectious diseases: 5/9% (candidiasis, HABP/VABP caused by Acinetobacter strains, Covid-19, RSV, catheter-related bloodstream infections)
• Metabolic disorders: 5/9% (type 2 diabetes, alpha-mannosidosis, Fabry disease, Pompe disease, primary hyperoxaluria type 1)
• Ophthalmology: 3/5% (dry eye disease, demodex blepharitis, GA secondary to AMD)
Number of drugs by regulatory pathway/designation (categories not mutually exclusive):
• Accelerated approval: 9
• Orphan drug designations: 18
• Fast track designation: 16
• Priority review: 24
• Breakthrough therapy: 9
Apologies for the suboptimal quality. If anyone wants a higher-resolution pdf copy, feel free to reach out!
FDA/CBER approvals to follow.”
Source: Daniel Neumeier/LinkedIn
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
