Lili Wang, Professor in the Department of Systems Biology at City of Hope, shared on X about recent paper by Prajish Iyer et al., titled “MGA deletion leads to Richter’s transformation by modulating mitochondrial OXPHOS” published on Science Translational Medicine.
Authors: Prajish Iyer, Bo Zhang, Tingting Liu, Meiling Jin, Kevyn Hart, Jibin Zhang, Viola Siegert, Marianne Remke, Xuesong Wang, Lei Yu, Joo Song, Girish Venkataraman, Wing C. Chan, Zhenyu Jia, Maike Buchner, Tanya Siddiqi, Steven T. Rosen, Alexey Danilov, Lili Wang.

“In our paper out today in Science Translational Medicine, we established a novel murine model of Richter Syndrome through MGA deletion. This work was lead by Dr. Prajish Iyer who is also the CLL Society 2024 Young Investigator Award recipient.
Big thanks to our amazing collaborators City of Hope including Danilov Lab, the Jia lab UC Riverside, Girish Venkataraman from The University of Chicago, Maike Buchner, TU Muenchen, and our wonderful editorial team Science Translational Medicine!
Richter syndrome is an aggressive transformation of CLL and remains an unmet clinical need. Here, we established a novel murine model to model the transition from CLL to RT by B-cell-restricted knockout of Mga, a negative regulator of MYC, in Cd19Cre-Cas9-Mdr-Sf3b1K700E mice.
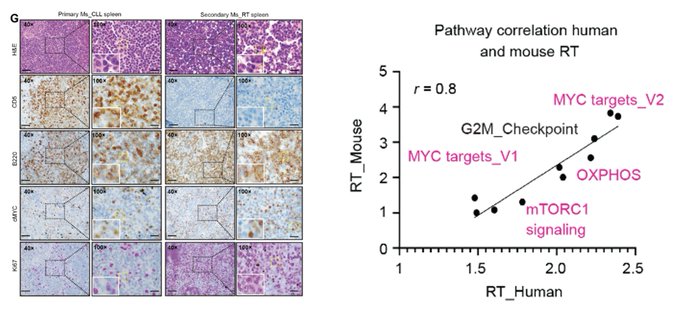
RT cells are transplantable and have high MYC and Ki67 expression, resembling molecular features of human RT cells.
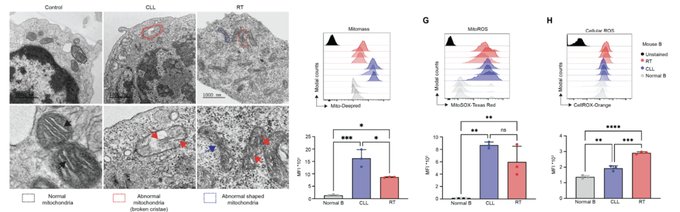
Interestingly, RT cells display significant mitochondrial structure changes, along with high Mitomass, MitoROS, and Cellular ROS, and OXPHOS.
Studies on human cell lines reveal that MGA KO leads to cell growth advantage, dependency on OXPHOS, and electron transport chain (ETC) protein complex II upregulation through MGA-NME1 regulatory axis.
We next targeted MYC and OXPHOS with AZ5576 (a CDK9 inhibitor) and TTFA (an ETC complex II inhibitor) in RT. Both compounds demonstrated significant therapeutic benefits in vivo, particularly in combinations, highlighting OXPHOS as a viable therapeutic target in RT.
Overall, our study establishes a new murine RT transplantable model, and suggests that the Mga-Nme1 axis drives murine CLL-to RT transition via modulation OXPHOS, emphasizing a potential therapeutic avenue for RT.“
Source: Lili Wang/X