Talha Badar: Outcome of MDS progressing on hypomethylating agent
Talha Badar, Hematologist/Oncologist at Mayo Clinic in Jacksonville, Florida, shared a post on X:
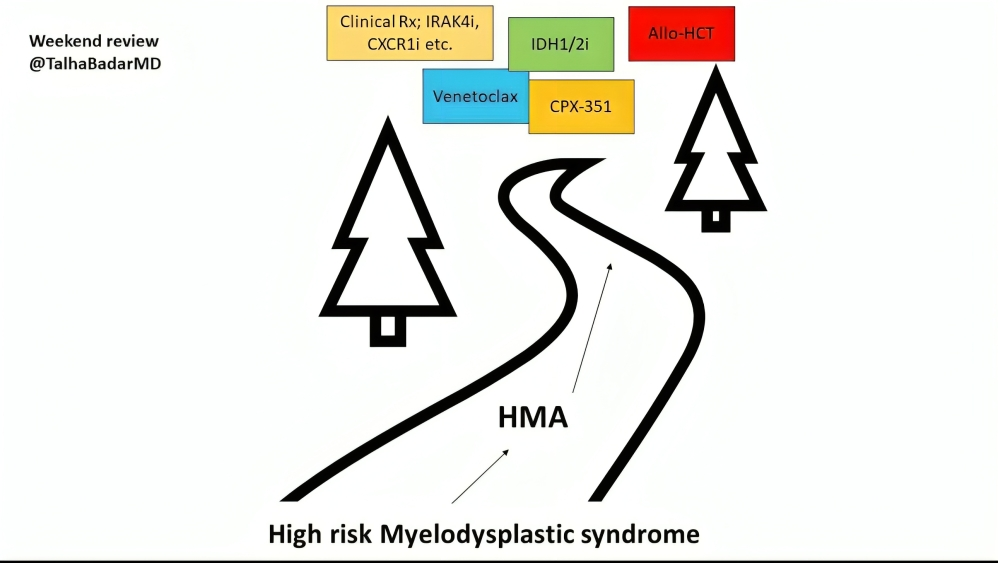
“Weekend review:
Outcome of MDS progressing on hypomethylating agent has dismal OS in the range of 6-9 months; worse with TP53m
No approved therapy for this poor prognostic disease.
Link to article.
Thoughts on prospective and retrospective studies.
MDS post HMA
Driver mutation in MDS progressing on HMA
TP53, ASXL1, RUNX1, and STAG2 (Ogawa et al. Blood 2019)
MDS post HMA
Inspire trial: PIII trial of rigosertib (PI3k/ PLK inhibitor) vs physicians choice.
Primary endpoint was OS, study failed to meet PE
MDS post HMA
ASTRAL-3 trial
Guadecitabine (novel s/c HMA which results in extended decitabine exposure) showed no difference in OS; 9.1 vs. 8.3 mo in physicians’ choice gp, p 0.61),
nor added clinically meaningful difference in DOR, LFS or TI.
June 2022 – Volume 6 – S3, EHA2022 Hybrid Congress Abstract Book
MDS post HMA
TAKEAIM LEUKEMIA: Emavusertib: IRAK4 and FLT3i
Splicing mt, drive overexpression of IRAK4 disease progression and poor prognosis.
PI/II study, no DLT, 57% mCR in HR-MDS pts with splicing factor mt
tested in combination AZA+VEN.
MDS post HMA
Omacetaxine was tested in MDS/CMML post HMA in PII study, showed ORR 33% and mOS 7.5 mo!
Better with diploid CG.
MDS post HMA
RWD, multi-center study of VEN+HMA post HMA showed reasonable ORR 59% and 62% -> allo-HCT.
Poor responders were TP53m and complex CG.
MDS post HMA
The idiome phase 2 study by the GFM group; evaluated IDHi in MDS failing HMA or HR-MDS.
32 pts treated, ORR was 69%, CR 46%.
DOR 7.4 mo, OS 14 mo (91% response rate in treatment naive HR-MDS)
MDS post HMA
CXCR1/2 Inhibitor (SX-682) in PI study showed single agent activity, more pronounce with splicing factors mutations.
MDS post HMA
PI/II study of low dose CPX-351 in MDS post HMA. Based on 2023 IWG response rate 59%. mOS 12.6 months.
MDS post HMA, summary slide below

Source: Talha Badar/X
