
Immunotherapy for Gastrointestinal Cancer
Introduction
Gastrointestinal cancers are a diverse group of malignancies that affect the digestive system, including the esophagus, stomach, liver, gallbladder, pancreas, small intestine, colon, rectum, and anus. These cancers collectively represent a significant portion of the global cancer burden, accounting for approximately 26% of all cancer cases and 35% of cancer-related deaths worldwide. The major types of gastrointestinal (GI) cancers include colorectal cancer, gastric cancer, esophageal cancer, liver cancer, and pancreatic cancer, each with distinct risk factors, clinical presentations, and treatment challenges.
The prevalence of GI cancers is influenced by various factors, including dietary habits, lifestyle choices, genetic predispositions, and infections. For instance, colorectal cancer is often linked to dietary and lifestyle factors, while liver cancer is closely associated with chronic hepatitis B and C infections. Despite advancements in early detection and treatment, the prognosis for many GI cancers remains poor, particularly for those diagnosed at advanced stages. The rising incidence of these cancers, especially among younger populations, underscores the need for effective prevention strategies, early detection, and innovative treatment approaches to improve patient outcomes.
Learn about the key symptoms and risk factors of stomach cancer in this video from Pancare Foundation.
Gastrointestinal Cancer Treatment Options
The treatment of GI cancers involves a multidisciplinary approach tailored to the specific type and stage of cancer, as well as the patient’s overall health and preferences. The primary treatment modalities include surgery, chemotherapy, radiation therapy, targeted therapy, and immunotherapy. Each of these treatments can be used alone or in combination to achieve the best possible outcomes for patients.
Treatment Options
Surgery
- Gastrectomy: Removal of part or all of the stomach, often used for stomach cancer. It can be a partial gastrectomy (removing part of the stomach) or a total gastrectomy (removing the entire stomach) to eliminate the tumor and nearby lymph nodes.
- Colectomy: Removal of part or all of the colon, commonly used for colorectal cancer. This procedure can be a partial colectomy (removing the cancerous section) or a total colectomy (removing the entire colon) to prevent cancer spread.
- Pancreaticoduodenectomy (Whipple procedure): Removal of the head of the pancreas, part of the small intestine, and other nearby structures, used for pancreatic cancer to remove the tumor and improve survival rates.
Chemotherapy
- Systemic Chemotherapy: Uses drugs like fluorouracil (5-FU), oxaliplatin, and cisplatin to kill cancer cells throughout the body. It can be used before surgery (neoadjuvant) to shrink tumors or after surgery (adjuvant) to eliminate remaining cancer cells.
- Regional Chemotherapy: Delivers high doses of chemotherapy directly to the area where the cancer is located, such as hepatic arterial infusion for liver cancer, to maximize the drug’s effect on the tumor while minimizing systemic side effects.
Radiation Therapy
- External Beam Radiation Therapy (EBRT): Uses high-energy X-rays to target and kill cancer cells. It can be used before surgery to shrink tumors or after surgery to destroy remaining cancer cells, and is also used for palliative care to relieve symptoms.
- Intensity-Modulated Radiation Therapy (IMRT): A more precise form of EBRT that modulates the radiation dose to conform to the shape of the tumor, minimizing damage to surrounding healthy tissues.
Targeted Therapy
- Monoclonal Antibodies: Drugs like trastuzumab (for HER2*-positive gastric cancer) and cetuximab (for EGFR*-positive colorectal cancer) target specific proteins on cancer cells to inhibit their growth and spread.
- Tyrosine Kinase Inhibitors: Drugs like imatinib and regorafenib block enzymes involved in cancer cell signaling pathways, used for gastrointestinal stromal tumors and metastatic colorectal cancers.
*Mutations of these genes may lead to increased sensitivity to growth factors with subsequent uncontrollable cellular division and cancerogenesis.
Each treatment option has its own set of benefits, risks, and success rates, which vary depending on the cancer’s stage and the patient’s overall health. A multidisciplinary team of healthcare professionals, including oncologists, surgeons, radiologists, and supportive care specialists, work together to create individualized treatment plans tailored to each patient’s needs.
The treatment options for gastric cancer are discussed in this video prepared by Johns Hopkins Medicine.
Immunotherapy for Gastrointestinal Cancer
Immunotherapy has emerged as a revolutionary treatment approach for various cancers, including GI malignancies. Unlike traditional chemotherapy and radiation therapy, which indiscriminately target rapidly dividing cells, immunotherapy aims to stimulate the body’s immune system to recognize and eliminate cancer cells more effectively.
The rationale behind using immunotherapy for GI cancers lies in the understanding that these tumors can evade immune surveillance and create an immunosuppressive tumor microenvironment. By modulating the immune system, immunotherapies can potentially overcome these barriers and enhance the anti-tumor immune response. Several types of immunotherapies have been explored for the treatment of GI cancers, including immune checkpoint inhibitors, adoptive cell transfer therapies, and cancer vaccines.
Immune Checkpoint Inhibitors
Immune checkpoint inhibitors are monoclonal antibodies that target specific proteins on immune cells, such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), and its ligand PD-L1. These proteins act as “brakes” on the immune system, preventing it from mounting an effective anti-tumor response. By blocking these checkpoints, immune checkpoint inhibitors can unleash the body’s immune cells to recognize and attack cancer cells more effectively.
Immune checkpoint inhibitors have shown promising results in various GI cancers, particularly in microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) tumors, which are characterized by a high mutational burden and increased immunogenicity.
Adoptive Cell Transfer Therapies
Adoptive cell transfer therapies involve isolating and expanding a patient’s immune cells, such as T cells or natural killer (NK) cells, and then reinfusing them back into the patient after ex vivo manipulation or genetic modification. One of the most promising adoptive cell transfer approaches is chimeric antigen receptor (CAR) T-cell therapy, where T cells are genetically engineered to express a synthetic receptor that recognizes specific tumor-associated antigens.
CAR-T cell therapies have shown remarkable success in hematological malignancies and are being actively investigated for solid tumors, including GI cancers. Ongoing research is focused on identifying suitable tumor-associated antigens and overcoming the challenges posed by the immunosuppressive tumor microenvironment in solid tumors.
Cancer Vaccines
Cancer vaccines aim to stimulate the immune system to recognize and mount a targeted response against tumor-associated antigens. Various types of cancer vaccines have been explored, including peptide vaccines, whole-cell vaccines, and dendritic cell vaccines.
While cancer vaccines have shown limited success as monotherapies in GI cancers, they are being investigated in combination with other immunotherapies or standard treatments, such as chemotherapy or radiation therapy, to enhance their efficacy.
Approved Immunotherapy Drugs for Gastrointestinal Cancer
Several immunotherapy drugs have been approved by regulatory agencies for the treatment of GI cancers, primarily targeting the PD-1/PD-L1 pathway:
- Pembrolizumab (Keytruda): A PD-1 inhibitor approved for the treatment of:
- MSI-H* or dMMR* solid tumors, including colorectal cancer and other GI cancers.
- Advanced gastric or gastroesophageal junction adenocarcinoma, in combination with chemotherapy or trastuzumab (for HER2-positive tumors).
- Advanced esophageal or gastroesophageal junction carcinoma, in combination with chemotherapy.
- Nivolumab (Opdivo): A PD-1 inhibitor approved for:
-
- MSI-H or dMMR colorectal cancer.
- Advanced gastric or gastroesophageal junction adenocarcinoma, in combination with chemotherapy.
- Esophageal or gastroesophageal junction cancer with residual disease after chemoradiotherapy.
- Ipilimumab (Yervoy): A CTLA-4 inhibitor approved in combination with nivolumab for the treatment of MSI-H or dMMR colorectal cancer.
- Dostarlimab (Jemperli): A PD-1 inhibitor approved for the treatment of advanced or recurrent endometrial cancer with dMMR, which includes some GI cancers with dMMR.
*Microsatellite instability-high (MSI-H) and mismatch repair deficient (dMMR)
These immunotherapy drugs have shown promising efficacy in selected patient populations, particularly those with MSI-H or dMMR tumors, which are more immunogenic and responsive to immune checkpoint inhibition.
Immunotherapy Benefits and Side Effects
Benefits of Immunotherapy for Gastrointestinal Cancer
Immunotherapy has demonstrated several potential benefits in the treatment of GI cancers:
- Improved survival outcomes: Clinical trials have shown that immunotherapies, particularly immune checkpoint inhibitors, can significantly improve overall survival in certain subgroups of GI cancer patients, such as those with MSI-H or dMMR tumors.
- Durable responses: Unlike traditional chemotherapy, which often provides temporary responses, immunotherapies can induce durable and long-lasting responses in some patients, potentially leading to long-term remission or even cure.
- Fewer side effects: Compared to chemotherapy, immunotherapies generally have a more favorable side effect profile, with fewer severe toxicities such as nausea, vomiting, and myelosuppression.
- Improved quality of life: Due to the potential for durable responses and fewer severe side effects, immunotherapies can contribute to an improved quality of life for GI cancer patients.
- Synergistic effects with other treatments: Immunotherapies can be combined with other treatment modalities, such as chemotherapy, radiation therapy, or targeted therapies, potentially enhancing their efficacy through synergistic effects.
Side Effects of Immunotherapy for Gastrointestinal Cancer
While immunotherapies have shown promising results in GI cancers, they can also cause unique side effects known as immune-related adverse events (irAEs). These side effects arise from the overstimulation of the immune system, leading to inflammation and autoimmune-like reactions in various organs and tissues. Common irAEs associated with immunotherapy in GI cancers include:
- Gastrointestinal toxicities: Diarrhea, colitis, and other gastrointestinal complications are among the most common irAEs observed with immunotherapies in GI cancers. These side effects can range from mild to severe and potentially life-threatening.
- Endocrine disorders: Immunotherapies can cause inflammation in endocrine glands, leading to conditions such as hypothyroidism, hyperthyroidism, adrenal insufficiency, and hypophysitis.
- Skin toxicities: Rashes, pruritus (itching), and other skin-related side effects are frequently reported with immunotherapies.
- Hepatic toxicities: Immunotherapy can cause liver inflammation (hepatitis), which can lead to elevated liver enzymes and, in severe cases, liver failure.
- Pulmonary toxicities: Pneumonitis (inflammation of the lungs) is a potentially serious side effect of immunotherapies, which can cause shortness of breath, cough, and respiratory distress.
- Musculoskeletal toxicities: Immunotherapies can cause inflammation in the joints (arthritis) and muscles (myositis), leading to pain, stiffness, and weakness.
- Neurological toxicities: Although rare, immunotherapies can cause inflammation in the brain, spinal cord, or peripheral nerves, leading to neurological complications such as encephalitis, meningitis, or neuropathies.
Managing Side Effects of Immunotherapy for Gastrointestinal Cancer
Effective management of irAEs is crucial for successfully implementing immunotherapies in GI cancers. Here are some strategies for managing side effects:
- Early recognition and monitoring: Regular monitoring and prompt recognition of irAEs are essential for timely intervention and prevention of severe complications. Patients should be educated about potential side effects and advised to report any new or worsening symptoms promptly.
- Grading and treatment guidelines: Various guidelines have been developed by professional organizations, such as the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN), to grade the severity of irAEs and provide recommendations for their management.
- Immunosuppressive therapy: Depending on the severity of the irAE, immunosuppressive medications, such as corticosteroids (e.g., prednisone) or other immunomodulatory agents (e.g., infliximab, mycophenolate mofetil), may be used to suppress the overactive immune response and control inflammation.
- Supportive care: Supportive care measures, such as hydration, electrolyte replacement, and symptomatic management, are essential for managing irAEs, particularly those affecting the gastrointestinal system.
- Multidisciplinary approach: The management of irAEs often requires a multidisciplinary team approach, involving oncologists, gastroenterologists, endocrinologists, dermatologists, and other specialists, depending on the affected organ systems.
- Treatment interruption or discontinuation: In cases of severe or life-threatening irAEs, temporary or permanent discontinuation of immunotherapy may be necessary to prevent further complications.
- Patient education and close monitoring: Patients should be educated about the potential side effects of immunotherapy and the importance of reporting any new or worsening symptoms promptly. Close monitoring and regular follow-up are essential for early detection and management of irAEs.
By implementing these strategies, healthcare professionals can optimize the benefits of immunotherapies while minimizing the risks and managing side effects effectively, ultimately improving patient outcomes and quality of life.
More information about completed and ongoing clinical trials for immunotherapy can be found here – clinicaltrials.gov.
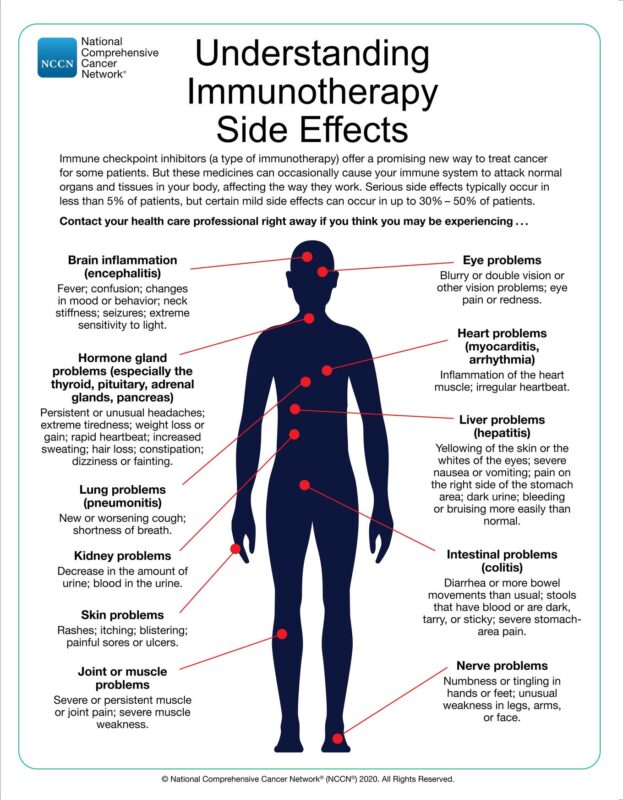
The image is taken from the National Comprehensive Cancer Network website.
Sources
- Gastrointestinal Cancers – American Cancer Society
- Stomach Cancer – American Society of Clinical Oncology
- Targeted Immunotherapies in Gastrointestinal Cancer: From Molecular Mechanisms to Implications – Frontiers in Immunology
- Recent FDA Approvals in Gastrointestinal Cancer – The ASCO Post
- Current status of immunotherapy in gastric cancer – Holistic Integrative Oncology
- GI Cancer Treatment – National Cancer Institute
- Recent Advances in Targeted Therapies for Advanced Gastrointestinal Malignancies – Cancers
