
Lorenz Mayr: The benefits of CAR-T therapy largely outweigh the potential risks of the CAR-T technology
Lorenz Mayr, CEO at Mayr BioMedTech Consulting, shared on LinkedIn:
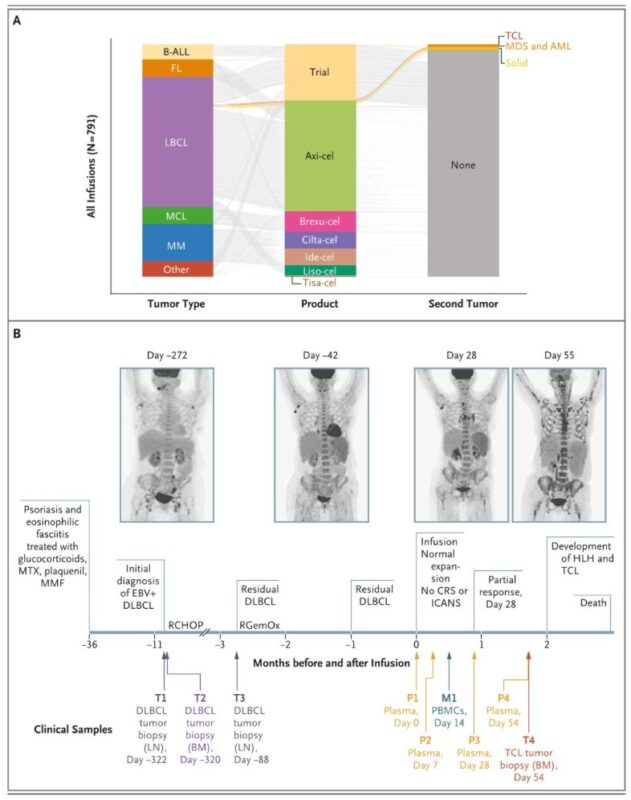
“Secondary Cancer Risk Is Low After CAR-T Cell Treatment:
A large study led by Stanford Medicine and published in NEJM has found that CAR-T cell therapies carry only a low risk of secondary malignancies.
The results come from a review of 724 patients who had undergone CAR-T therapies between 2016 and 2024. Overall, the study found that secondary cancers arose in approximately 6.5% of patients over a median follow-up period of three years. According to the publication, this incidence rate was ‘roughly similar’ to patients who had been treated instead with stem cell therapy.
The study identified one patient who died due to a secondary T-cell cancer, though Stanford researchers contend this was likely caused by the immunosuppression associated with CAR-T therapies, rather than a mis-insertion of the gene for the CAR construct during the preparation of the treatment
The data from Stanford Medicine confirm data from a similar study at the Unversity of Pensylvania Medical Center, published earlier this year.
It needs to be stated that the benefits of CAR-T therapy largely outweigh the potential risks of the CAR-T technology.
Original publications:
Risk of Second Tumors and T-Cell Lymphoma after CAR T-Cell Therapy
NEJM
Mark P. Hamilton et al.
T-Cell Cancer after CAR T-Cell Therapy
NEJM
Emily Mitchell et al.
Indolent CD4+ CAR T-Cell Lymphoma after Cilta-cel CAR T-Cell Therapy
NEJM
Metin Ozdemirli et al.
Secondary Cancers Following CAR T Cell Therapy Are Rare, Penn Medicine Analysis Shows
Penn Medicine
Meagan Raeke et al.
Background reading:
Secondary Cancer Risk Is Low After CAR T Cell Treatment: Stanford Study
BioSpace
Tristan Manalac
FDA Requires Boxed Warning for Secondary Cancers on CAR-T Therapies
BioSpace
Tristan Manalac
The Fate(s) of CAR T-Cell Therapy: Navigating the Risks of CAR+ T-Cell Malignancy
AACR
Mohamed Abou-el-Enein.”
Source: Lorenz Mayr/X
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
