Photo from Gautam Mehta/X
Jul 19, 2024, 22:19
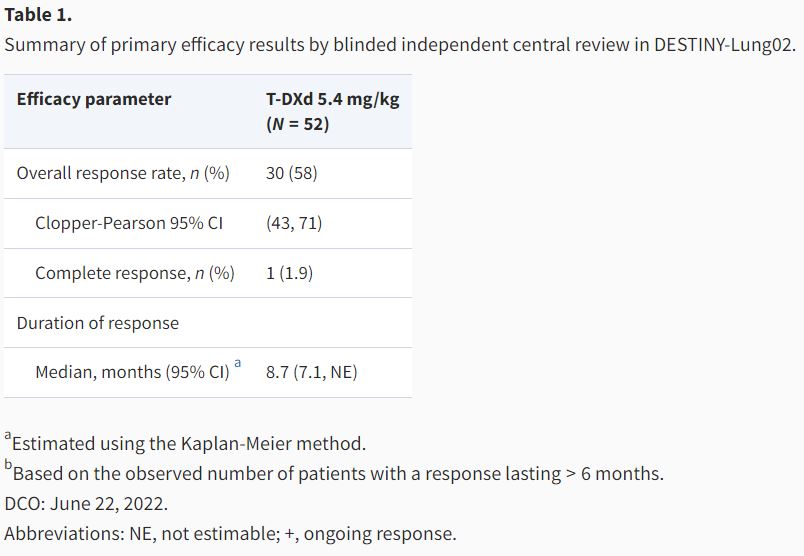
Gautam Mehta summarizes the FDA accelerated approval of fam-trastuzumab deruxtecan-nxki for HER2-mutated NSCLC
FDA oncology shared on X:
“OCE’s Gautam Mehta and colleagues summarize the FDA accelerated approval of fam-trastuzumab deruxtecan-nxki—the first targeted treatment option for patients with HER2-mutated non-small cell lung cancer – via The Oncologist Journal.”
FDA approval summary: fam-trastuzumab deruxtecan-nxki for unresectable or metastatic non-small cell lung cancer with activating HER2 mutations
Autors: Gautam U Mehta, Paz J Vellanki, Yi Ren, Anup K Amatya, Pallavi S Mishra-Kalyani, Lili Pan, Jeanne F Zirkelbach, Yuzhuo Pan, Jiang Liu, Stephanie L Aungst, Claudia P Miller, Mirat Shah, Nam Atiqur Rahman, Marc Theoret, Paul Kluetz, Richard Pazdur, Julia A Beaver, Harpreet Singh
Source: FDA Oncology/X
Anup K Amatya
cancer
Claudia P Miller
FDA Oncology
Gautam Mehta
Gautam U Mehta
Harpreet Singh
HER2 gene
HER2 mutant NSCLC
Jeanne F Zirkelbach
Jiang Liu
Julia A Beaver
Lili Pan
Marc Theoret
Mirat Shah
Nam Atiqur Rahman
OncoDaily
Oncology
Pallavi S Mishra-Kalyani
Paul Kluetz
Paz J Vellanki
Richard Pazdur
Stephanie L Aungst
The Oncologist Journal
Yi Ren
Yuzhuo Pan
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
Nov 14, 2024, 12:14
Nov 14, 2024, 12:09
Nov 14, 2024, 11:56
Nov 14, 2024, 11:49
Nov 14, 2024, 11:46
Nov 14, 2024, 11:38
Nov 14, 2024, 11:30
Nov 14, 2024, 09:31
Nov 14, 2024, 09:26