
New Paper Alert! Novel Tool Predicts Chemotherapy Response in Pancreatic Cancer Using AI-assisted Transcriptomic Signatures
Novel Tool Predicts Chemotherapy Response in Pancreatic Cancer Using AI-assisted Transcriptomic Signatures
Authors: Nicolas Fraunhoffer, Pascal Hammel, Thierry Conroy, Rémy Nicolle, Jean-Baptiste Bachet,Alexandre Harlé, Vinciane Rebours, Anthony Turpin, Meher Ben Abdelghani, Emmanuel Mitry, James J Biagi, Brice Chanez, Martin Bigonnet , Anthony Lopez, Ludovic Evesque, Thierry Lecomte, Eric Assenat, Olivier Bouché, Daniel J Renouf, Aurélien Lambert, Laure Monard, Marc Mauduit, Jérôme Cros, Juan Iovanna, Nelson Dusetti.
Published in Annals of Oncology on June 18, 2024 .
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is an extremely aggressive cancer with poor survival rates even after surgical resection and adjuvant chemotherapy. While the PRODIGE-24 trial showed that modified FOLFIRINOX (mFFX) improves outcomes over gemcitabine in the adjuvant setting, mFFX also has higher toxicity.
Currently, the choice between these two main adjuvant regimens is based largely on patient performance status rather than considering the underlying tumor biology and predicted chemosensitivity. To enable more personalized treatment selection, this study developed the Pancreas-View tool integrating artificial intelligence (AI)-optimized transcriptomic signatures specifically designed to predict sensitivity to gemcitabine, 5-FU, irinotecan, and oxaliplatin in PDAC.
When applied to samples from the PRODIGE-24 trial, Pancreas-View accurately identified patients most likely to benefit from their assigned treatment. Among those receiving mFFX, patients predicted as sensitive had a median disease-free survival of 50 months versus only 13.9 months for resistant patients. Similarly, for gemcitabine, sensitive patients achieved 33.7 month median disease-free survival compared to 9.6 months in resistant cases.
Nearly half the patients were deemed sensitively “matched” to their treatment, with substantially improved outcomes. In contrast, patients classified as “unmatched” or “all resistant” had very poor survival regardless of regimen. By integrating AI-driven transcriptomics with preclinical chemotherapy data, Pancreas-View enables biomarker-guided selection of optimal adjuvant therapy for resected PDAC.
Design/Methods
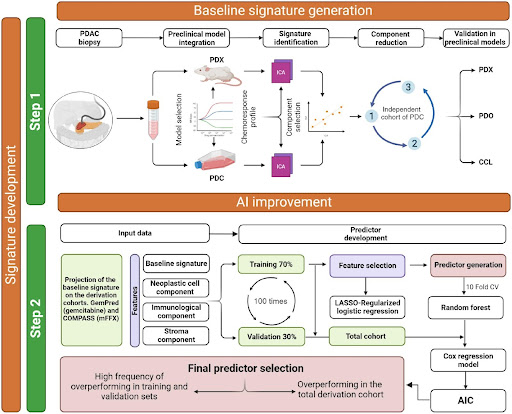
- The researchers developed baseline transcriptomic signatures for key PDAC drugs (gemcitabine, 5-FU, irinotecan, oxaliplatin) using preclinical models of chemotherapy response.
- Using machine learning on independent patient cohorts (GemPred and COMPASS), they optimized these baseline signatures by incorporating transcripts of master regulators and microenvironment features associated with drug response into “AI signatures”.
- The AI signatures were integrated into the Pancreas-View tool which predicts chemosensitivity for each agent.
- Pancreas-View was clinically validated using 343 patients from the phase 3 PRODIGE-24/CCTG PA6 trial randomized to gemcitabine or mFFX adjuvant therapy after resection.
What We Learned
- In PRODIGE-24, patients predicted sensitive by Pancreas-View to their assigned treatment had significantly longer disease-free survival (DFS) and cancer-specific survival (CSS) than resistant patients.
- For mFFX, the median DFS was 50.0 months in sensitive vs 13.9 months in resistant patients (HR 0.39, p<0.001).
- For gemcitabine, the median DFS was 33.7 months in sensitive vs 9.6 months in resistant patients (HR 0.25, p<0.001).
- Patients whose treatment matched their Pancreas-View prediction had markedly better outcomes than unmatched patients or those resistant to all drugs.
Key Highlights
- 47.8% of patients were sensitively matched, with median DFS of 50.1 months for mFFX and 33.7 months for gemcitabine.
- 25.1% were unmatched and 27.1% were resistant to all drugs, both with median DFS of only ~10 months.
- The AI signatures remained independently predictive in a multivariate analysis adjusting for clinicopathologic factors.
- Pancreas-View integrates transcriptomics with preclinical models and machine learning to predict chemosensitivity.
Key Takeaway Messages
This study presents the Pancreas-View tool incorporating transcriptomic AI signatures from preclinical models to predict sensitivity to gemcitabine accurately and mFFX chemotherapy in resected PDAC patients. Using Pancreas-View to guide treatment selection could significantly improve disease-free and overall survival while minimizing toxicity from ineffective regimens.
With prospective validation, this biomarker-driven approach could enable personalised adjuvant therapy and optimize outcomes for PDAC patients undergoing surgery.
Summary by Amalya Sargsyan, MD
