
New Paper Alert! Liposomal Irinotecan vs Topotecan for Relapsed Small Cell Lung Cancer: RESILIENT Phase 3 Trial
Liposomal Irinotecan vs Topotecan for Relapsed Small Cell Lung Cancer: RESILIENT Phase 3 Trial
Authors: David R. Spigel, Afshin Dowlati, Yuanbin Chen, Alejandro Navarro, James Chih-Hsin Yang, Goran Stojanovic, Maria Jove, Patricia Rich, Zoran G. Andric, Yi-Long Wu, Charles M. Rudin, Huanyu Chen, Li Zhang, Stanley Yeung, Fawzi Benzaghou, Luis Paz-Ares
Published in the Journal of Clinical Oncology on April 22, 2024
Introduction:
Small cell lung cancer (SCLC) is a highly aggressive form of lung cancer characterized by rapid doubling time and early metastases. Despite initial response to first-line platinum-based chemotherapy, most patients experience relapse within 1-2 years, highlighting the need for effective second-line treatment options. The phase 3 RESILIENT trial aimed to compare the efficacy and safety of liposomal irinotecan, a novel liposomal formulation of irinotecan designed to prolong drug exposure, with the standard second-line therapy topotecan in patients with relapsed SCLC after progression on or following first-line platinum-based chemotherapy.
Study Design and Methods:
- Randomized, open-label, phase 3 trial enrolling 461 patients with SCLC
- Eligibility: Radiologically confirmed disease progression on/after first-line platinum-based chemotherapy, ECOG PS 0-1, life expectancy >12 weeks
- Randomization 1:1 to:
- Liposomal irinotecan 70 mg/m2 IV every 2 weeks (6-week cycle)
- Topotecan 1.5 mg/m2 IV daily for 5 days, every 3 weeks (6-week cycle)
- Primary endpoint: Overall Survival (OS)
- Key secondary endpoints: Progression-Free Survival (PFS) per BICR, Objective Response Rate (ORR) per BICR, safety
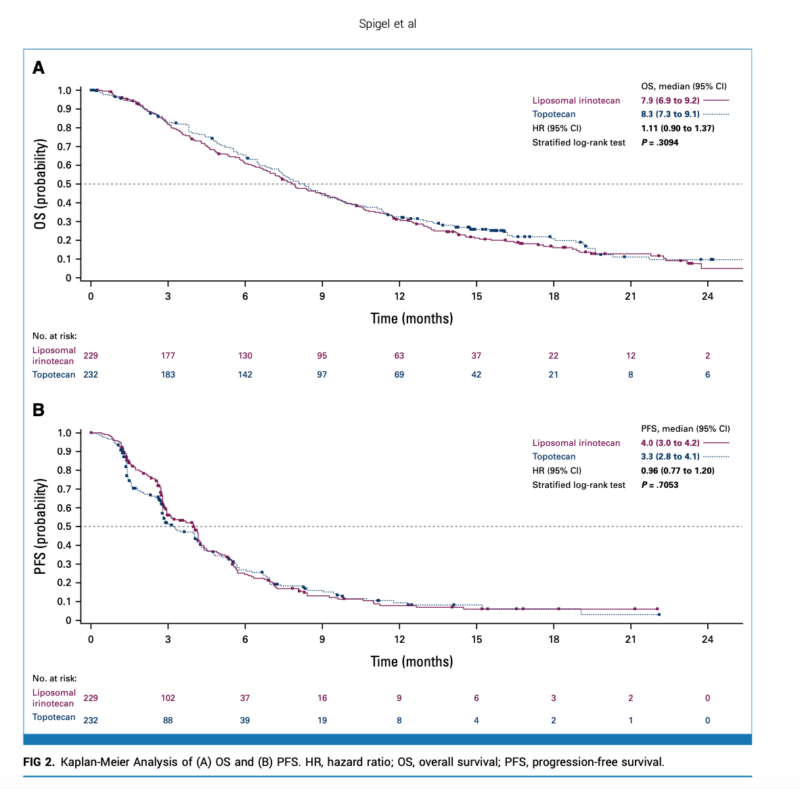
Results:
- Median OS: 7.9 months (liposomal irinotecan) vs. 8.3 months (topotecan); HR 1.11 (95% CI 0.90-1.37), P=0.31
- Median PFS per BICR: 4.0 months (liposomal irinotecan) vs. 3.3 months (topotecan); HR 0.96 (95% CI 0.77-1.20), nominal P=0.71
- ORR per BICR: 44.1% (liposomal irinotecan) vs. 21.6% (topotecan), nominal P<0.0001
- Complete/partial responses: 5.2%/38.9% (liposomal irinotecan) vs. 3.0%/18.5% (topotecan)
- Grade ≥3 Treatment-Emergent Adverse Events (TEAEs): 62.4% (liposomal irinotecan) vs. 87.9% (topotecan)
- Liposomal irinotecan: diarrhea (13.7%), neutropenia (8.0%), decreased neutrophil count (4.4%)
- Topotecan: neutropenia (51.6%), anemia (30.9%), leukopenia (29.1%), thrombocytopenia (29.1%)
Key Highlights:
- Liposomal irinotecan doubled ORR compared to topotecan (44.1% vs. 21.6%), with non-overlapping 95% CIs.
- Lower incidence of grade ≥3 TEAEs and TEAE-related discontinuations with liposomal irinotecan vs. topotecan.
- The safety profile of liposomal irinotecan is consistent with the known profile, and diarrhea is the most frequent grade ≥3 related to TEAE.
Conclusions:
- While the primary endpoint of OS was not met, liposomal irinotecan demonstrated similar PFS and a significantly higher ORR compared to topotecan in patients with relapsed SCLC after progression on or following first-line platinum-based chemotherapy.
- The favorable safety profile of liposomal irinotecan, with a lower incidence of severe toxicities than topotecan, supports its potential as an effective and well-tolerated second-line treatment option for patients with relapsed SCLC.
- These findings provide a rationale for further investigating liposomal irinotecan, potentially in combination with other therapies, to improve treatment outcomes in the relapsed SCLC setting.
Summary By Amalya Sargsyan, MD
