
New Paper Alert! The INNOVATED Cohort: The Role of Immune Checkpoint Blockers in Solid Organ Transplant Recipients with Cancer
The INNOVATED Cohort: The Role of Immune Checkpoint Blockers in Solid Organ Transplant Recipients with Cancer
Authors: J. Remon, E. Auclin, L. Zubiri, S. Schneider, D. Rodriguez-Abreu, N. Minatta, O. Gautschi, F. Aboubakar, E. Muñoz-Couselo, T. Pierret, S.I. Rothschild, F. Cortiula, K.L. Reynolds, C. Thibault, A. Gavralidis, N. Blais, F. Barlesi, D. Planchard, B.M.D. Besse
Introduction:
Solid organ transplant (SOT) recipients have an increased risk of developing cancer due to chronic immunosuppressive therapy. However, these patients are typically excluded from clinical trials evaluating immune checkpoint blockers (ICBs) due to concerns about potential allograft rejection and the impact of immunosuppressive drugs on ICB efficacy.
Design/Methods:
The INNOVATED (ImmuNe checkpoint inhibitors outcome solid organ transplant recipients with cancer eVAluated in an inTErnational Database) cohort study is a multicenter, retrospective database collecting real-world data on the efficacy and safety of ICBs in Solid organ transplant recipients with advanced solid tumours. The study included 31 patients (98% kidney transplant recipients) treated with ICBs from August 2016 to October 2022. The primary endpoints were overall response rate (ORR), progression-free survival (PFS), overall survival (OS), and the rates of grade 3 immune-related adverse events (ir-AEs) and allograft rejection.
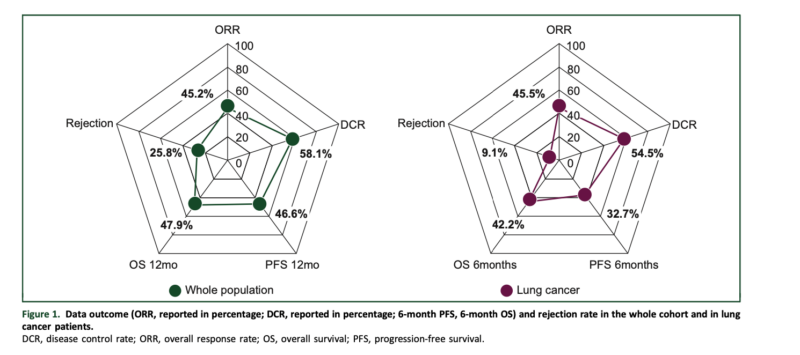
What We Learned:
In the overall cohort, the ORR was 45.2%, with a 6-month PFS of 53.8% and a 6-month OS of 62.7%. The grade 3 ir-AE rate leading to ICB discontinuation was 12.9%, comparable to the general population. However, the allograft rejection rate was 25.8%, with 88% resulting in allograft loss.
In the non-small cell lung cancer (NSCLC) subgroup (n = 11), the ORR was 45.5%, with a 6-month PFS of 32.7% and a 6-month OS of 42.2%. The allograft rejection rate in this subgroup was 9.1%.
Key Highlights:
- ICB therapy demonstrated promising efficacy in SOT recipients with advanced solid tumors, particularly in the overall cohort.
- The risk of allograft rejection was significant, occurring in up to one-third of patients within the first 2 months of ICB initiation.
- The allograft rejection risk was independent of prior rejection history, response to ICB, ICB strategy (monotherapy or combination), and modifications to immunosuppressive therapy.
- In the NSCLC subgroup, the PFS and OS outcomes were lower than those reported in non-transplant patients, potentially due to the transitory effect of ICBs, poorer prognosis of lung cancer in SOT recipients, and higher immunosuppression.
Key Takeaway Messages:
- ICBs could be considered a feasible option for Solid organ transplant recipients with life-threatening advanced malignancies and no other therapeutic alternatives where ICB treatment has been associated with tumour response.
- However, the decision to use ICBs in SOT recipients should be individualized, considering the potential risk of allograft rejection and the need for close monitoring, especially within the first 2 months of ICB initiation.
- Multidisciplinary collaboration with transplant experts is crucial for careful patient selection, tailoring of immunosuppressive therapy, and monitoring strategies.
- Further research is needed to identify the specific subgroups of SOT recipients with solid tumours who would benefit most from ICB treatment and to optimize the management strategies for this patient population.
Summary by Amalya Sargsyan, MD
Immune checkpoint blockers in solid organ transplant recipients and cancer: the INNOVATED cohort
