
Agenus and Tanner Pharma Partner to Expand Global Access to BOT/BAL
Agenus and Tanner Pharma announced a collaboration to provide expanded access to botensilimab (BOT) and balstilimab (BAL).
Through a Named Patient Program (NPP), this initiative provides patients with microsatellite stable colorectal cancer (MSS CRC) and other advanced solid tumors the chance to receive BOT/BAL based on clinical evidence and medical need.
Named Patient Program Provides Access to Botensilimab and Balstilimab for patients with Advanced Solid Tumors and MSS Colorectal Cancer
The NPP allows patients, in collaboration with their doctors, to use BOT/BAL before it receives regulatory approval, ensuring compliance with ethical and regulatory standards.
Tanner Pharma will manage access to BOT/BAL for patients in geographies that allow named patient access to investigational medicines. The NPP ensures that patients, in consultation with their physicians, can access BOT/BAL even before regulatory approval, adhering to all ethical and compliance standards.
BOT and BAL are experimental immunotherapies targeting difficult cancers, including MSS CRC and other tumors that are typically resistant to immune therapies.
Clinical studies have shown complete pathological responses in neoadjuvant MSS colon cancer patients and lasting tumor responses across various cancer types. These findings position BOT/BAL as a potential new treatment option that could change the approach to treating hard-to-treat cancers.
About Agenus

Agenus is a leading immuno-oncology company targeting cancer with a comprehensive pipeline of immunological agents. The company was founded in 1994 with a mission to expand patient populations benefiting from cancer immunotherapy through combination approaches, using a broad repertoire of antibody therapeutics, adoptive cell therapies (through MiNK Therapeutics) and adjuvants (through SaponiQx).
Agenus has robust end-to-end development capabilities, across commercial and clinical cGMP manufacturing facilities, research and discovery, and a global clinical operations footprint. Agenus is headquartered in Lexington, MA.
“Agenus is proud to announce a collaboration with Tanner Pharma Group to provide expanded access to our investigational immunotherapies through a Named Patient Program.
This program addresses urgent needs for eligible patients with microsatellite stable Colorectal Cancer (MSS CRC) and other advanced solid tumors by enabling access to our investigational treatments in regions where named patient access is permitted.”
About Tanner Pharma Group

Tanner Pharma Group focuses on providing global access solutions for specialty medications. Through Named Patient Programs, compassionate use, and expanded access programs, Tanner Pharma connects patients and healthcare providers with vital treatments, while maintaining high standards of ethical and regulatory compliance.
“Tanner Pharma is thrilled to announce our partnership with Agenus to expand access to botensilimab (BOT) and balstilimab (BAL) through a Named Patient Program (NPP).
This initiative aims to provide patients with microsatellite stable colorectal cancer (MSS CRC) and other advanced solid tumors the opportunity to access these investigational immunotherapies. BOT and BAL have shown promising clinical outcomes, including complete pathological responses and durable tumor responses across multiple cancer types.
This partnership underscores our commitment to supporting patients and healthcare providers by offering new hope for improved survival and quality of life.”
About Botensilimab
Botensilimab is a human Fc enhanced CTLA-4 blocking antibody designed to boost both innate and adaptive anti-tumor immune responses. Its novel design leverages mechanisms of action to extend immunotherapy benefits to “cold” tumors which generally respond poorly to standard of care or are refractory to conventional PD-1/CTLA-4 therapies and investigational therapies.
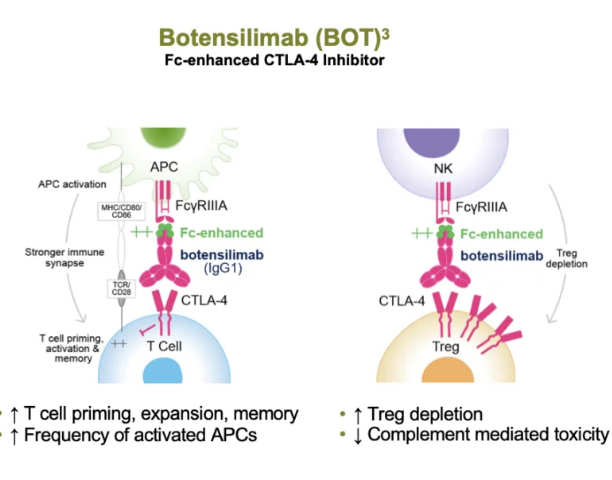
Botensilimab augments immune responses across a wide range of tumor types by priming and activating T cells, downregulating intratumoral regulatory T cells, activating myeloid cells and inducing long-term memory responses.
Approximately 1,100 patients have been treated with botensilimab in phase 1 and phase 2 clinical trials. Botensilimab alone, or in combination with Agenus’ investigational PD-1 antibody, balstilimab, has shown clinical responses across nine metastatic, late-line cancers.
Further Reading:
Three decades of pioneering in Immune Oncology – Agenus
Botensilimab: A Revolutionary Fc-Enhanced CTLA-4 Inhibitor Transforming Cancer Immunotherapy
Botensilimab’s mechanism of action and potential in addressing treatment-resistant cancers
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
