
Maximilian Strobl: Could adaptive therapy improve PARPi maintenance for ovarian cancer?
Maximilian Strobl, Postdoctoral Researcher at Moffitt Cancer Center and Research Institute, shared a post on X:
“Could adaptive therapy improve PARPi maintenance for ovarian cancer?
I’m very happy and proud to share our new paper, now out in Cell Systems.
A tweetorial.

Personalized medicine has established the idea of tailoring drugs to patients – but, what about personalizing dose, frequency, or duration of treatment?
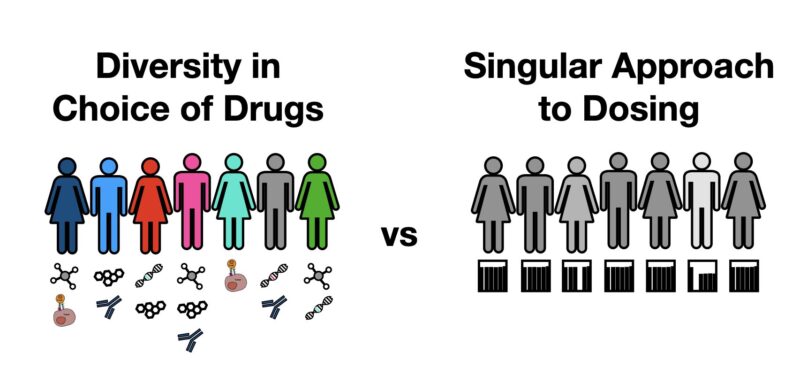
Benefit of PARPis for OVCA patients varies widely: Some are cured, but most relapse with varying (possibly limited) survival benefit. Yet, all get the same amount of drug, and struggle with the toxicity. What if we could better account for how much drug we really need?
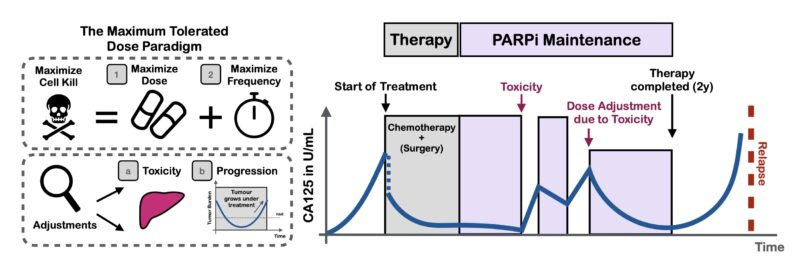
Here, we explored using adaptive therapy (AT) to personalize how we de-escalate treatment. AT uses tumor burden dynamics to iteratively adjust therapy and has shown promise in prostate cancer. But, how and when should we adapt? Should we skip treatments or modulate dose?
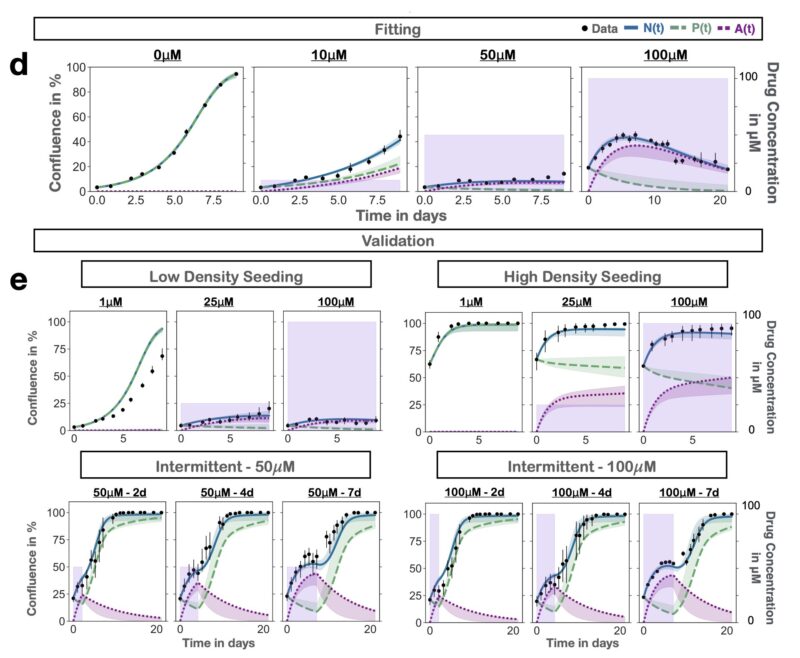
To address this question, we developed a mathematical model of the PARPi response dynamics. Our approach was built on two principles: 1) Iteration, and 2) Validation.
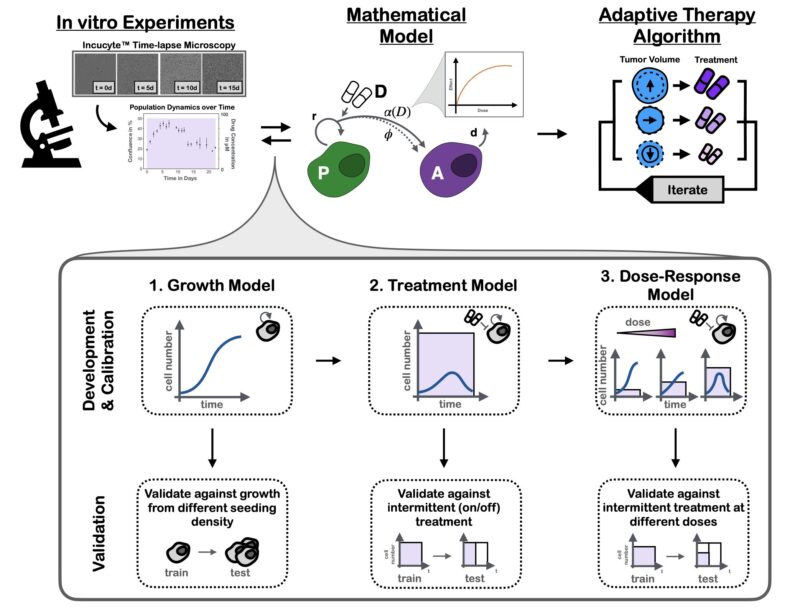
Iteration: at each step we tested multiple plausible hypotheses against each other using data from time-series microscopy. This strengthened confidence in the final model and allowed us to show that damaged cells continue to proliferate (P) for 1-2 times before arrest (A).

Validation: given we planned to use the model to make predictions about new treatment schedules, we validated that it could predict the dynamics to such unseen schedules. The final results are almost too good to be true – yet they are!
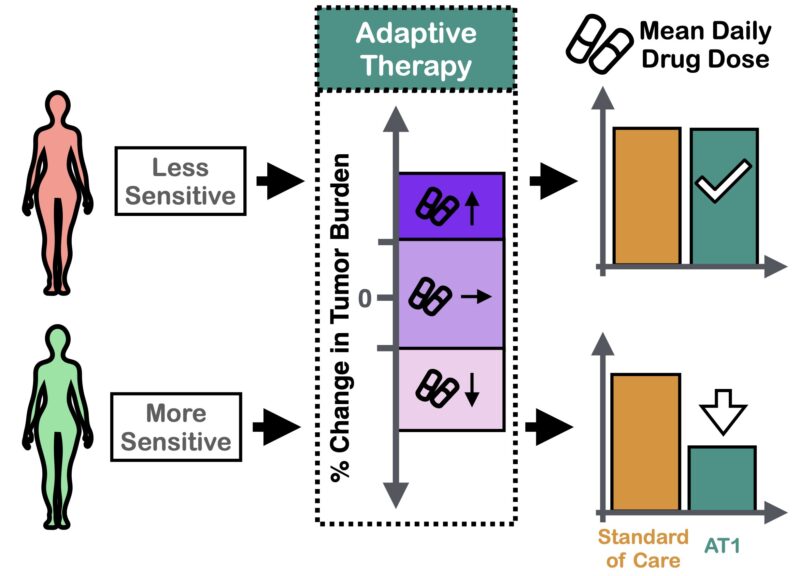
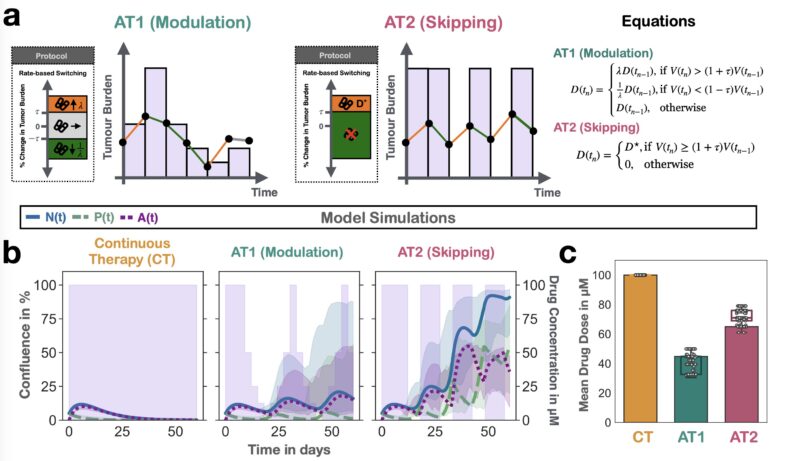
Next, we used the model to ask: can we reduce dose using adaptive therapy? The answer was “yes”, BUT only if we modulated dose. Pilot mouse experiments confirmed this conclusion.
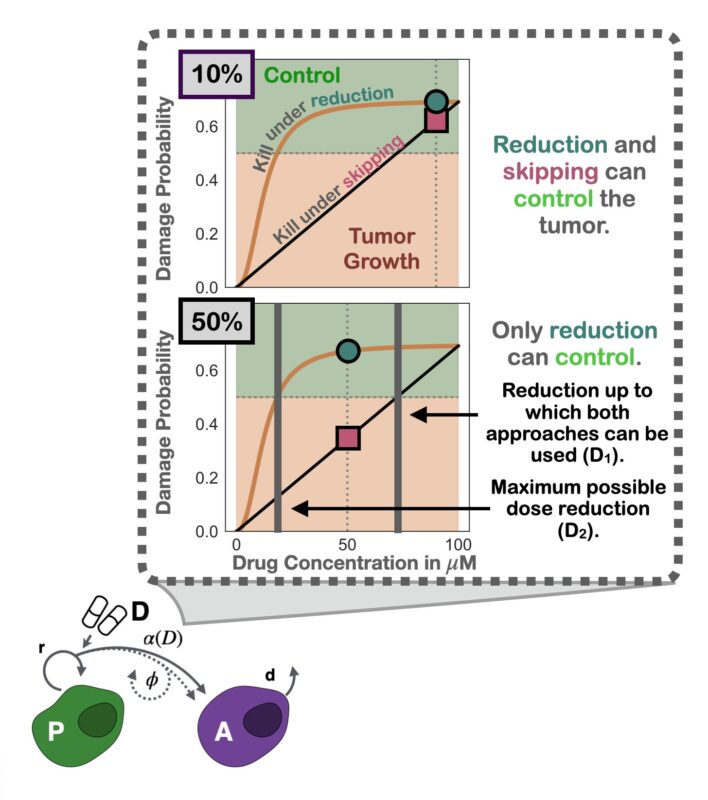
Nudged by helpful reviewers, we dug deeper: why was modulation better? The simple yet elegant answer: the curvature of the inferred dose response. Since it’s diminishing, skipping exerts less average growth suppression than modulation (Jensen’s inequality).
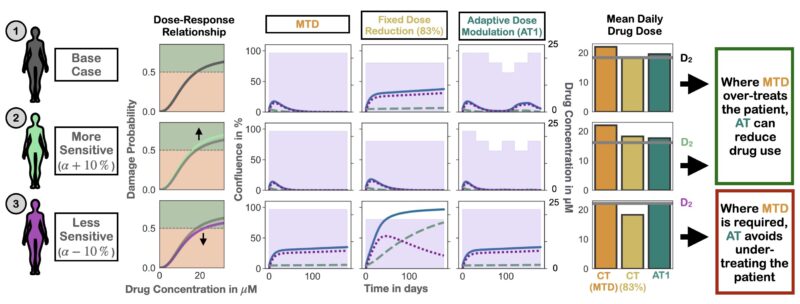
What does this mean for PARPi maintenance? De-escalating treatment for everyone is hard – some patients need MTD for tumor control. But if we use adaptive therapy and let the tumor burden dynamics guide us we can personalize de-escalation.
Many thanks to Damaghi laboratory (the CEELs) for introducing me to the wet laboratory, and to my fantastic mentors and colleagues on this project:
Sandy Anderson
Philip Maini
Robert Wenham
Alexandra Martin
Jeffrey West
Jill Gallaher
Mark R-T
The Gatenby Laboratory
Centre for Evolutionary Therapy
IMO
Oxford Mathematics
Moffitt Cancer Center
P.S. I believe preclinical testing of our assumptions and “real-world” proofing is crucial to advance AT – and holds many exciting questions. Read more here.”

Source: Maximilian Strobl/X
