
New Paper Alert! Complete Response with Chemoimmunotherapy in Intrahepatic Cholangiocarcinoma
Complete Response with Chemoimmunotherapy in Intrahepatic Cholangiocarcinoma
Authors: Matthew D. Robinson, Roseanna Wheatley, Lucy Foster, Saurabh Jamdar, Ajith K. Siriwardena, Angela Lamarca, Richard Hubner, Juan W. Valle, and Mairéad G. McNamara
Published in JCO Precision Oncology on April 25, 2024
Introduction:
Cholangiocarcinoma (CCA), a rare and aggressive form of liver cancer, has seen a global increase in incidence and mortality rates. The majority of patients present with advanced-stage disease, where palliative chemotherapy remains the only treatment option.
This case report highlights an exceptional outcome in a patient with metastatic intrahepatic cholangiocarcinoma (iCCA) exhibiting high tumour mutational burden (TMB) who achieved a complete pathological response after receiving a combination of chemotherapy and immunotherapy.
Case Details:
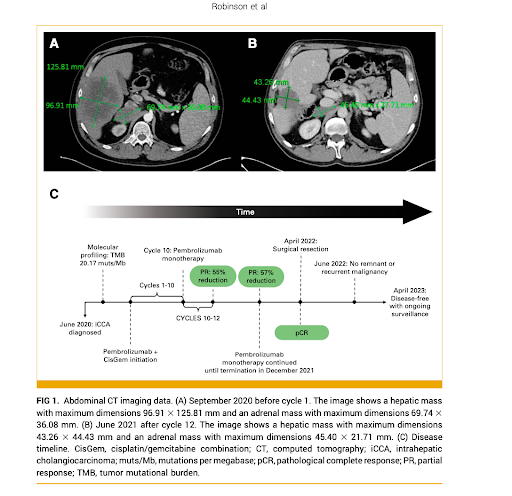
A 49-year-old male patient presented with abdominal pain and was diagnosed with intrahepatic cholangiocarcinoma with extrahepatic metastasis to the adrenal gland.
Molecular profiling revealed a high TMB of 20.17 mutations per megabase despite a stable microsatellite instability status. The patient was enrolled in a clinical trial and received 10 cycles of cisplatin/gemcitabine (CisGem) combined with pembrolizumab, an anti-PD-1 immunotherapy agent, followed by two cycles of pembrolizumab monotherapy.
Key Highlights:
- Radiological and biochemical evidence of partial response was observed after 12 treatment cycles, with normalization of tumour markers (CA 19-9, CEA, and CA 125) and a 55% decrease in the dimensions of the marker lesions.
- Treatment was well-tolerated, with only grade 1 toxicities initially. However, grade 3 alanine transaminase elevation led to permanent discontinuation of pembrolizumab.
- Multidisciplinary team discussion led to a decision for surgical intervention, and subsequent histological analysis of the surgical specimens revealed a complete pathological response, with no viable tumour tissue present.
- The patient remained disease-free, with a progression-free survival exceeding 38 months as of the latest follow-up in August 2023.
What We Learned:
- The Potential Role of Tumor Mutational Burden (TMB) as a Biomarker: While microsatellite instability status is considered the most reliable biomarker for predicting response to immunotherapy, this case demonstrates the potential benefit of TMB assessment in guiding treatment decisions for patients with advanced CCA.
- The Importance of Molecular Profiling: Early tumor molecular profiling, including TMB status, should be considered in patients with advanced CCA before initiating first-line treatment to identify suitable clinical trials or targeted therapies.
- This case highlights the importance of multidisciplinary team discussions at critical junctures in the disease course to facilitate adaptive changes in management strategies, ultimately leading to improved patient outcomes.
- The synergistic effect of combining chemotherapy (CisGem) with immunotherapy (pembrolizumab) was instrumental in achieving a complete pathological response in this case, emphasizing the potential of combination approaches in treating advanced CCA.
Key Takeaway Messages:
- TMB assessment should be incorporated into routine clinical genomic testing for patients with advanced CCA to identify potential candidates for immunotherapy-based treatments.
- Multidisciplinary team discussions and adaptive management strategies are crucial in optimizing treatment outcomes for patients with rare and aggressive cancers like CCA.
- Combination approaches integrating chemotherapy and immunotherapy may offer improved responses and potential curative opportunities in select cases of advanced CCA with high TMB.
- Further research and re-evaluation of global approvals for immunotherapy in treating TMBhigh tumors are warranted to expand access to potentially life-saving therapies
Summary by Amalya Sargsyan, MD
