
Yonghao Yu: PARP inhibitiors for SCLC
Yonghao Yu, Professor of Molecular Pharmacology and Therapeutics at Columbia University Irving Medical Center, shared a post on LinkedIn:
“PARP inhibitiors for SCLC! PARP1 as a therapeutic target for BRCAmut cancers has been well established. However, PARPi are increasingly being used for cancers without BRCA mutations. In this new publication, we sought to understand the mechanism of action of PARPi in SCLC.
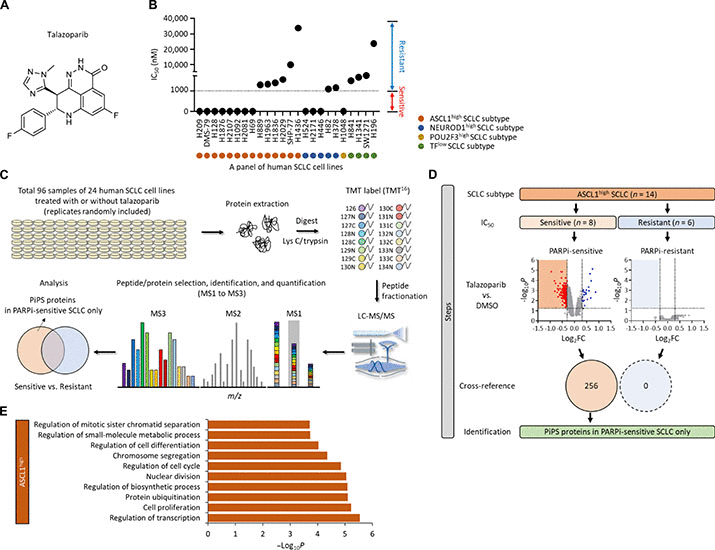
Small cell lung cancer (SCLC) is a highly malignant and recalcitrant cancer. In this paper, we assembled a large panel of 24 SCLC cell lines that represent the major SCLC subtypes, I.e., ASCL1+, NeuroD1+, Pou2F3+, etc.
We performed large scale quantitative proteomics experiments to characterize how the proteomes of these SCLC cell lines respond to Talazoparib. We found that talazoparib-sensistive SCLC cells undergo a major remodeling of their proteomes in response to Talazoparib treatment.
Among the down regulated proteins, we were particularly intrigued by ASCL1, which is a lineage specific oncoprotein for SCLC. ASCL1 plays an essential role in maintaining the neuroendocrine differentiation states of SCLC. Furthermore, ASCL1 is a key survival factor for SCLC.
Importantly, we found that ASCL1 is robustly degraded upon the treatment of not only PARPi, but also clinically relevant chemotherapeutic agents. This is an important discovery because of the well established sensitivity of SCLC to chemo.
How ASCL1 gets degraded in response to genotoxic stress? We found that a DNA damage-sensitive E3 ubiquitin ligase, Huwe1, is critically involved in this process. ASCL1 is a substrate of Huwe1, and Huwe1 targets ASCL1 for degradation under genotoxic conditions.
Interestingly, we found that even though PARPi treatment induces an universal DNA damage signal in all SCLC cell lines tested, these various SCLC cells respond to this DNA damage signal differently. In cells expressing Huwe1, ASCL1 is robustly degraded, leading to cell death.
In contrast, in SCLC cells that do not express Huwe1, ASCL1 is not degraded, and there is no cell death. As a result, Huwe1 expression could be a predictive biomarker for the therapeutic efficacy of PARPi in SCLC.
The biochemical effects of PARPi (e.g., PARP trapping and DNA damage) are general, regardless of cellular contexts. However, cells respond to this signal differently. Expression of lineage specific oncoproteins could be a novel synthetic leathality mechanism for PARP inhibitiors.
KUDOS to the first author, Dr. Chiho Kim, for leading this extraordinary study. I would also like to thank our wonderful collaborators, Drs. John Minna, Ben Drapkin, Han Liang, Ling Cai.”
Read further.
Source: Yonghao Yu/LinkedIn
