Salvador Santiesteban: Oligometastatic breast cancer and oligoprogression
Salvador Santiesteban, Medical oncologist from the National Institute of Medical Sciences and Nutrition, shared on X: .
“Oligometastatic breast cancer (OMBC) and oligoprogression. According to ESMO, ESTRO and ASTRO, the oligometastatic disease is defined by the presence of up to 5 lesions treatable with local therapy.
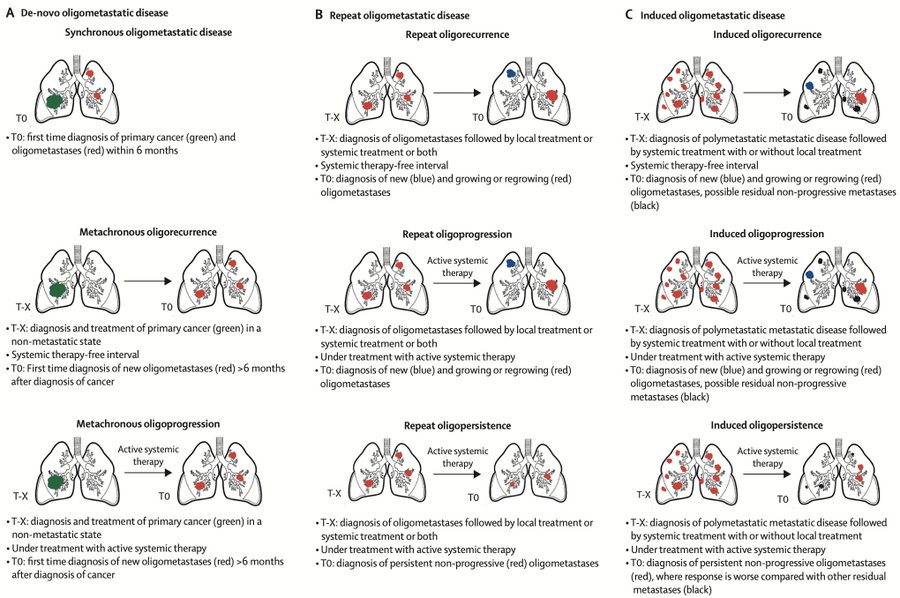
However, it is actually a much more complex entity that can be subclassified depending on the different stages and treatments throughout the patient’s natural history of the disease.
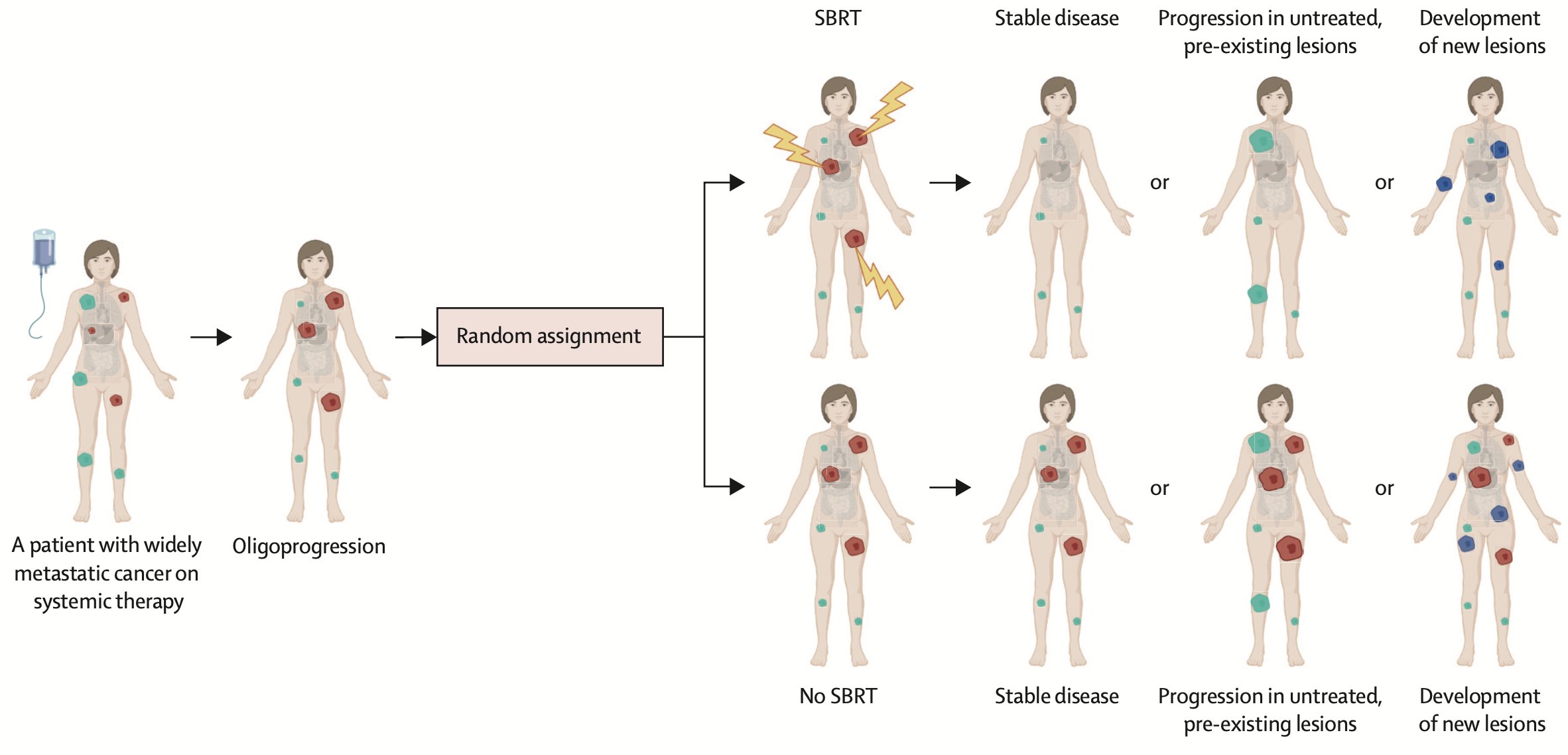
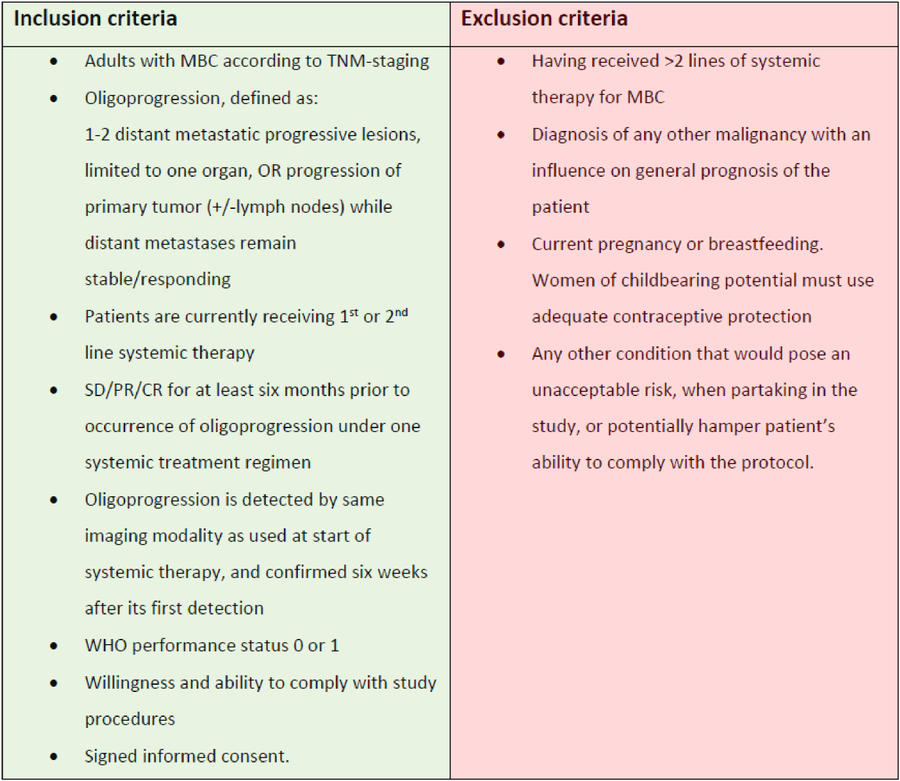
One of the major challenges when designing clinical trials for oligometastatic disease or oligoprogression in breast cancer is the biological differences that could arise from the various clinical scenarios of the disease (see attached image), in combination with the different molecular subtypes.
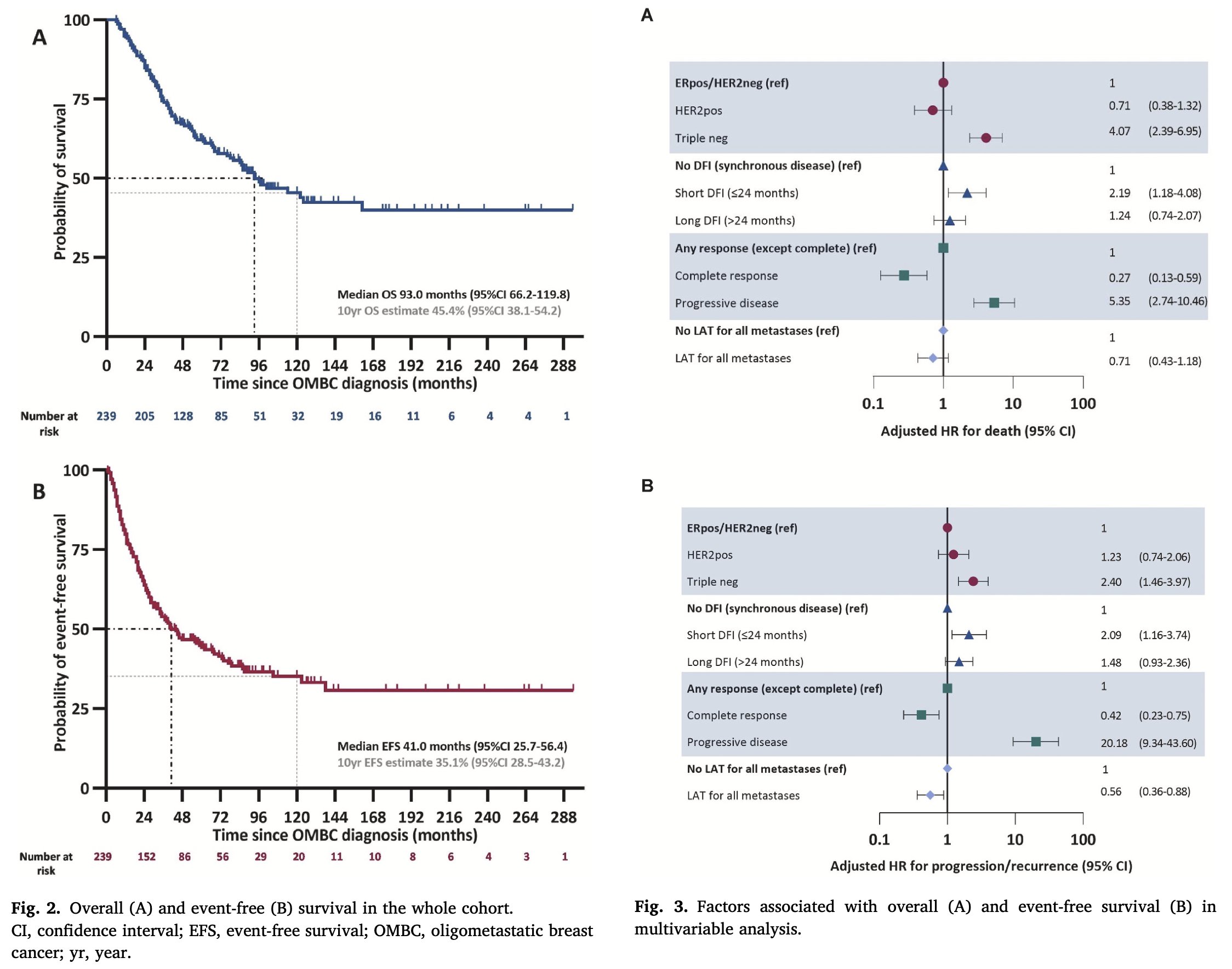
Prognosis: Multiple studies have consistently demonstrated improved overall survival in patients with de novo OMBC.
Is this indicative of a more indolent disease with slow growth and lower metastatic capacity. Could it suggest diagnosis at an earlier stage of the disease or perhaps the effectiveness of multimodal treatment (local therapy plus systemic therapy).
But what prospective information do we have regarding the treatment of OMBC?
- SABR-COMET
- NRG-BR002 trial
- BOSTON trial
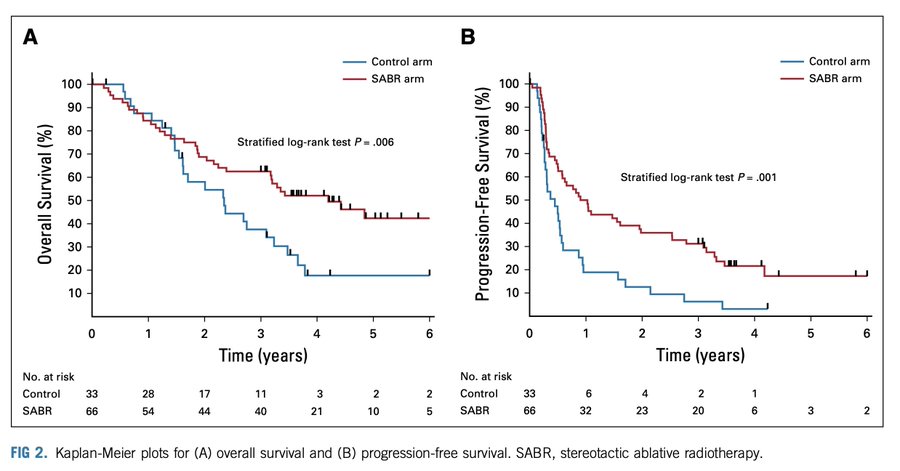
SABR-COMET Phase 2 trial:
1 – 5 metastatic lesions (max of 3 mets in any organ), primary tumor must have been treated definitively. 18 out of 99 randomized patients had a diagnosis of breast cancer.
Median progression free survival was 6∙0 months in the control group vs 12 months in the SABR group (HR 0∙47 p=0∙0012).
In the ITT population, median OS was 28 months in the control arm vs 50 months in the SABR arm (HR 0.47 p=0.006).
NRG-BR002 trial (Abstract):
1L OMBC (<4 extracranial mets) on systemic therapy (ST) for <12 months.
Randomized ST vs ST and local therapy.
N=125 79% ER+ or PR+/HER2-, 13% HER2+, 8% triple negative. 60% had 1 metastasis, 20% presented synchronously with primary disease, 93% received SBRT. PFS: ST 23 mo vs ST + LT 19.5 mo 36-mo PFS: ST 32.8% vs ST and LT 38.1% p=0.36.
Median OS was not reached in either arm 36-mo OS: ST 71.8% vs ST + LT 68.9% p = 0.54. The trial will not proceed to the Phase III component.
BOSTON trial: OMBC (1-3 bone metastases).
Control of primary tumor with surgery or RT Pre-treatment assesment with CT and Bone Scan followed by NaF PET/CT.
15 patients were treated at a dose of 20 Gy in 1 fx to each metastasis. 73% Luminal 20% HER2 positive 7% TNBC Local PFS at 2 yrs 100% PFS at 2 years 65% .
What about oligoprogression? There remains no definitive consensus regarding the precise number of metastases that would distinguish oligoprogression from more extensive disease progression, although the most common definition involves three or fewer, with some studies including patients with up to five sites of disease progression.
Treatment of oligoprogressive disease could preserve subsequent treatments for later administration and, therefore, enable a longer total duration of treatment and possibly even a longer survival time.
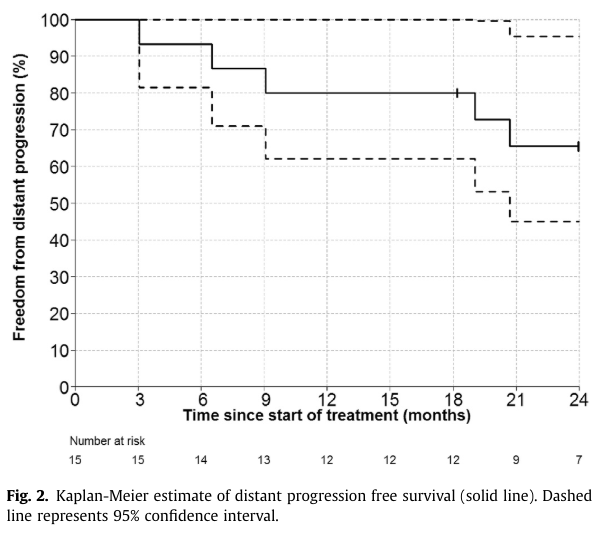
A retrospective analysis (F. Marazzi et al) included pts undergoing RT on up to three sites of oligoprogression with continuation of systemic therapy.
The primary endpoint was progression-free survival after RT (PFS-AR); the secondary endpoint was overall survival (OS). N= 27. PFS-AR 12.2 mo (95 CI 3-38 mo) OS was 53 months (95% CI 16-190 mo). No clinical factors were significantly associated with OS.
AVATAR is a phase 2 trial. Luminal MBC in 1-2L with CDK4/6i and AI >6m 1-5 extracranial oligoprogression lesions amenable to SABR Subsequent SABR was permitted to delay change in systemic therapy N= 32. mFU 15.8 months.
The most common sites of oligoprogresion were bone 44 (71%), and nodal 11 (18%). 47% of patients remained event free for ≥ 6 months. The median modified PFS was 10.4 months and 46% remained unchanged on systemic therapy for 12 months.
Median PFS was 5.2 months (95% CI: 3.1-6.8) 10/30 (33%) progressions suitable for a second course of SABR for oligoprogression to further delay systemic therapy change. No grade >3 toxicities reported.
The CURB phase 2 study: SBRT vs no SBRT in patients with oligoprogressive MBC or NSCLC. Defined as having progression in up to 5 individual lesions n= 47 breast cancer 59 NSCLC TNBC 16 pts (34% ) Non-TNBC 31 patients.
Benefit of SBRT in PFS 3.2 mo SOC vs 7.2 mo SBRT, driven by NSCLC. Breast cancer cohort: PFS SOC 4.2 months vs SBRT 4.4 mo (HR 0.78 p=0.43).
No difference by ER status. 29 (62%) patients with breast cancer who had progression after random assignment developed new lesions outside of the radiation field, regardless of treatment group assignment.
COntinue the SaMe systemic therapy after local ablative therapy for Oligoprogression in metastatic breast cancer – the COSMO study (ONGOING) Primary endpoint: PFS rate at six months (PFS-6). Secondary endpoints: PFS, OS, Time to next line of systemic therapy.
Primary and secondary endpoints will also be stratified by localization of progressive lesion and BC subtype (ER+/HER2- vs. HER2+ vs. TNBC).
CONCLUSIONS:
The OMBC has a better prognosis (possibly due to tumor biology, early detection, or multimodal treatment). It is unclear which biological subtype benefits most from local therapies for OMBC and what is the optimal timing for treatment (at diagnosis versus after a few months of stability).
The design of clinical trials for the treatment of oligoprogressive breast cancer is even more complex, and no specific group of patients has been identified to derive the greatest benefit from local therapy in this scenario.
There are many unanswered questions, so in the absence of conclusive evidence, the management of OMBC should always be made by a multidisciplinary team.”
Source: Salvador Santiesteban/X