
Charles Swanton: What is the role of the TME in lung cancer evolution?
Charles Swanton, Professor at shared on X/Twitter:
“What is the role of the TME in lung cancer evolution? We are excited to share our new TRACERx paper ‘Spatial Architecture of Myeloid and T Cells Orchestrates Immune Evasion and Clinical Outcome in Lung Cancer’ at AACR24, out in Cancer Discovery.
Visit the article website.
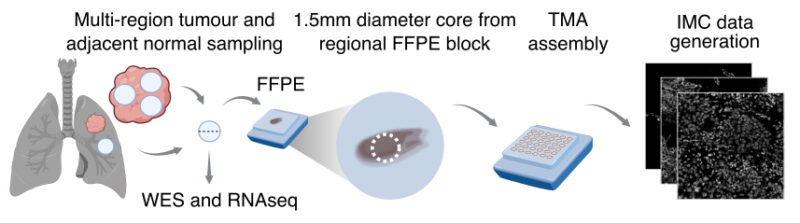
2/19 We integrated imaging mass cytometry (IMC), clinical and genomics (WES, RNAseq) data to decipher links between spatial TME organisation, tumour immunogenicity and tumour evolutionary history across multiple non-small cell lung cancer subtypes.
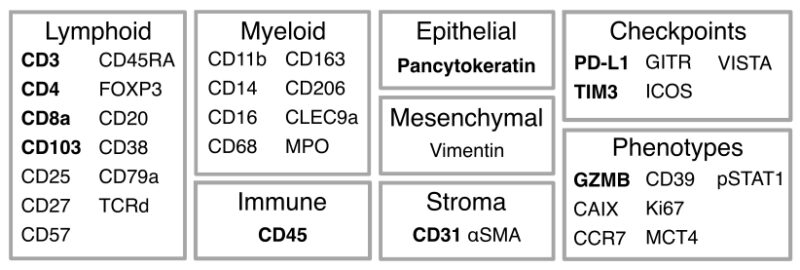
3/19 Using IMC, we profiled in situ expression of 38 markers on tumour and adjacent normal lung samples acquired at surgery. A pan-immune antibody panel targeted innate and adaptive immune cells while a T cells and stroma panel probed T cell states and stromal cells.
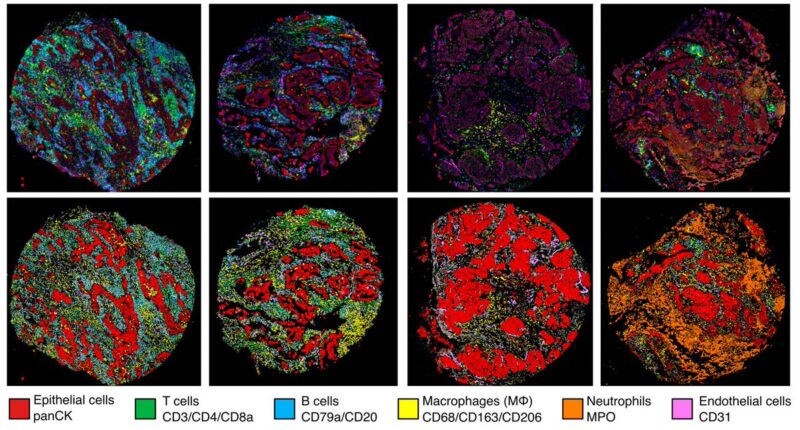
4/19 We identified 29 immune cell types and epithelial, endothelial and aSMA cells (2.3 million cells/panel) in 198 tumour regions from 81 treatment-naive early stage lung cancer patients. These cells recurrently organised into 10 pan-histology communities.
5/19 By quantifying immune cell types within tumour nest and stroma areas, we identified four histology-independent TME classes, distinguished by differential cell densities of TILs, macrophages and neutrophils.
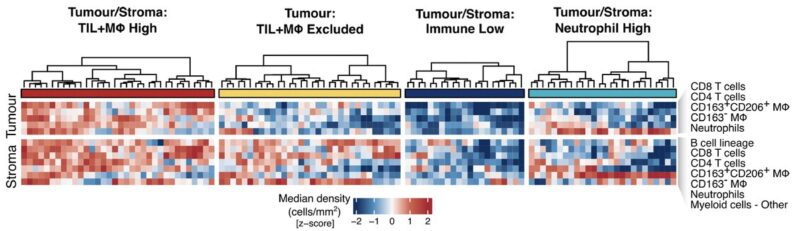
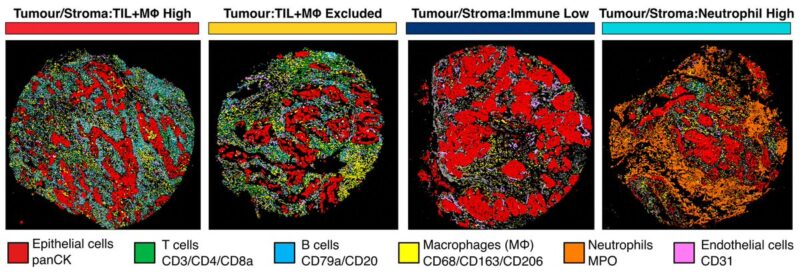
6/19 Previous definitions of TME architectures have been based on TILs alone (hot, excluded, cold). We expanded to include myeloid cells and identified a fourth class with low TIL and high neutrophil infiltrate (TS:Neutrophil High) in 19% of tumour cores.
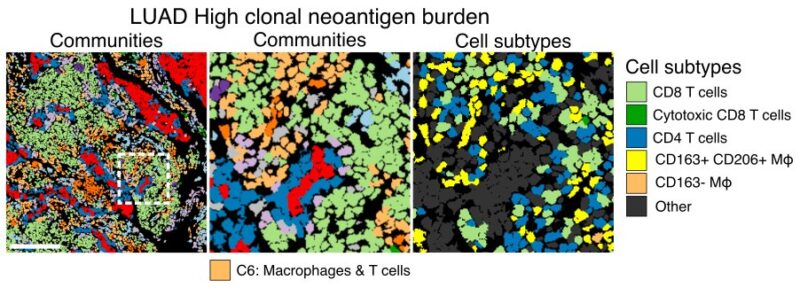
7/19 Having derived our single cell atlas, we first asked how TME organisation changes with neoantigen burden. In LUAD, spatial niches of T cells and macrophages increased in density with increasing clonal neoantigen burden and were associated with intrinsic immune evasion.
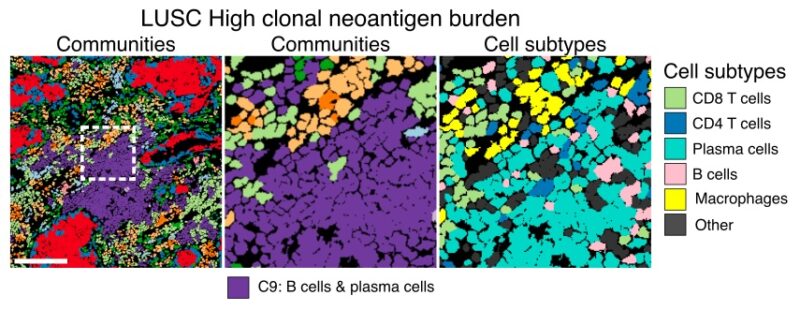
8/19 Conversely, niches of plasma and B cells increased with clonal neoantigen burden in LUSC. This community was not associated with intrinsic immune escape but was enriched in excluded TMEs, suggesting spatial barriers to immune predation.
9/19 Building on studies suggesting a role for CAFs in immune exclusion, we explored whether our TME classes were associated with distinct aSMA CAF arrangements and found that immune-low TMEs were associated with peritumoral CAF barriers to CD8 T cell infiltration.
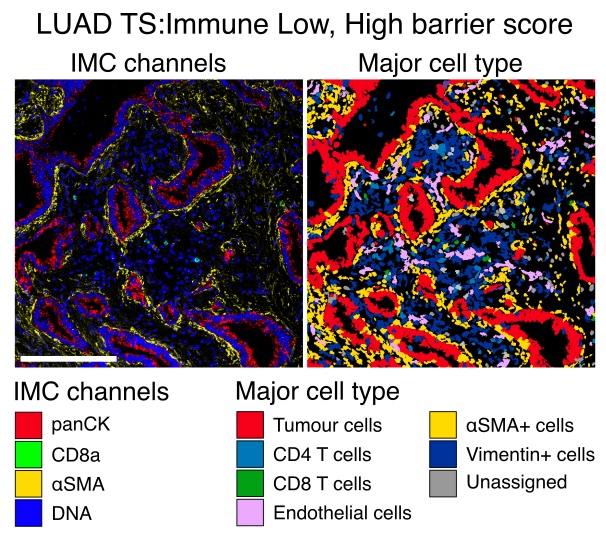
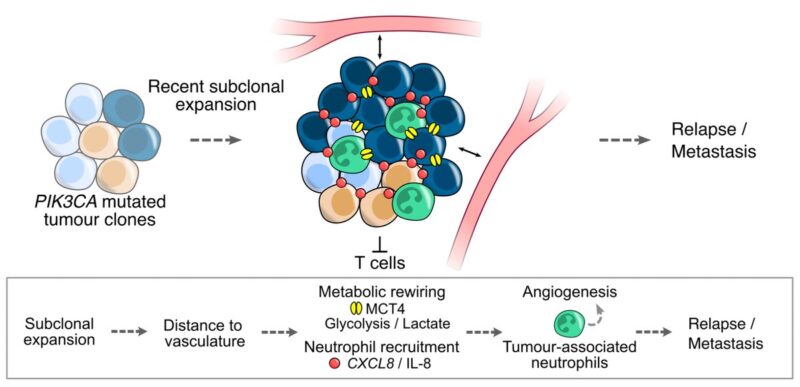
10/19 Our work revealed a TME with high neutrophil densities and sparse TIL infiltration in the tumour nest and stroma, TS: Neutrophil High. Given previous associations between neutrophils and mortality in NSCLC, we investigated potential tumour-promoting roles of this class.
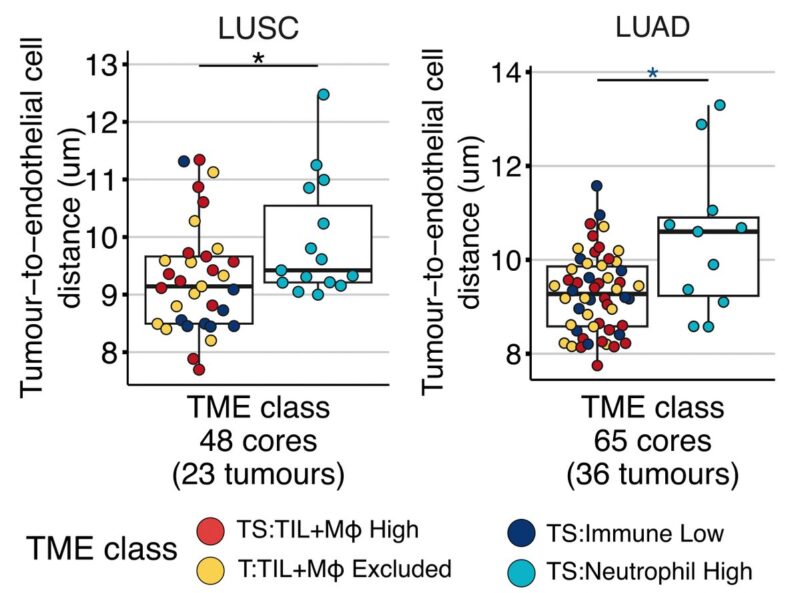
11/19 Data suggested metabolic reprogramming of tumours in this class compared to others. Neutrophil high tumours were characterised by greater distances between tumour cells and vasculature than in other classes, and by increased tumoral MCT4 protein expression in LUSC.
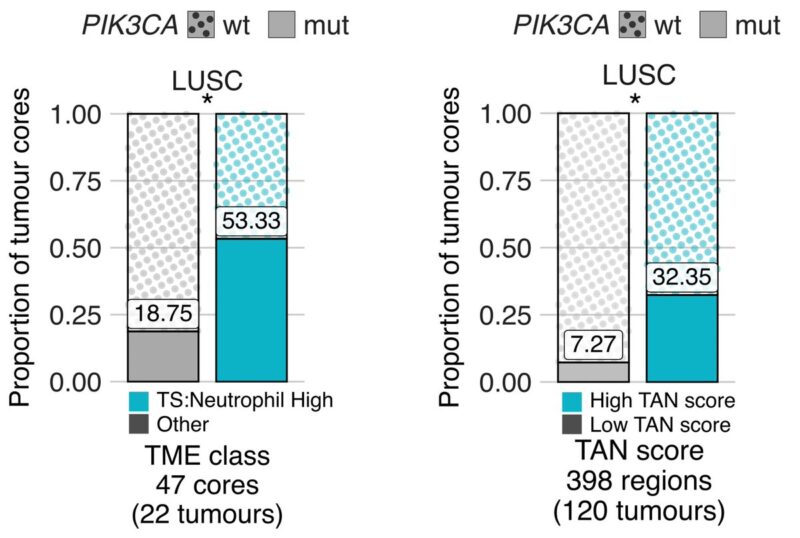
12/19 We set out to dissect genomic aberrations in neutrophil high TMEs that may enhance fitness. Using IMC and tumour-associated neutrophil (TAN) scoring from H and Es, in LUSC, we found that PIK3CA driver mutations were more frequently observed in neutrophil-rich TMEs.
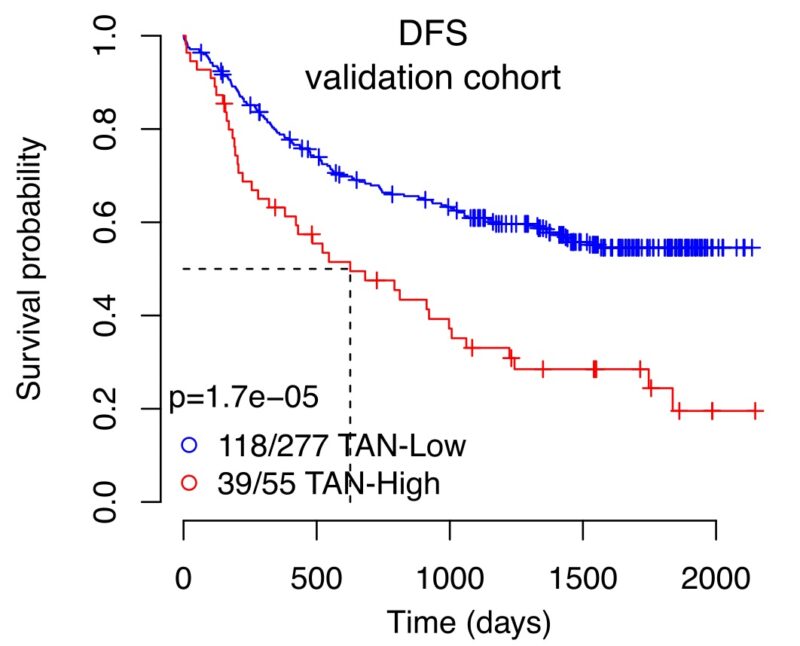
13/19 To conclude, we investigated whether neutrophil infiltration was linked to risk of disease relapse and metastasis in NSCLC. TAN-High tumours had a shorter disease-free survival than TAN-Low tumours independent of stage in TRACERx and TCGA cohorts.
14/19 Finally, building on TRACERx work identifying recent subclonal expansions as prognostic indicators, we observed larger expansions in TAN-High than TAN-Low tumours, associating neutrophil-rich TMEs with evolutionary dynamics of poor outcomes.
Visit the article website.
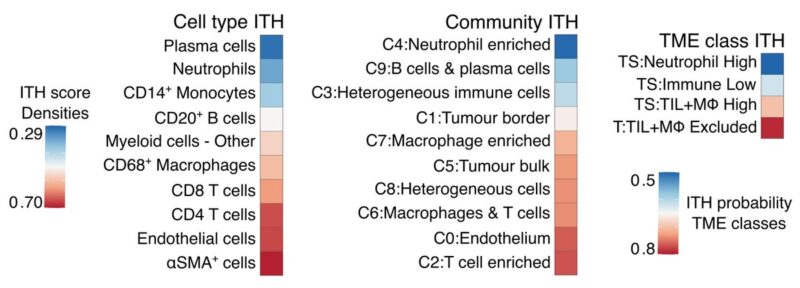
15/19 Low intratumour heterogeneity (ITH) of biomarkers or targets is vital for their clinical applicability. Notably, neutrophils, their spatial community, and TME class had the lowest spatial ITH, together with plasma cells, compared to other immune cell types and communities.
16/19 Altogether, this study provides insights into the spatial organisation of the lung cancer TME in the context of tumour immunogenicity and cancer evolution, describes TME ITH and barriers to immune surveillance, and implicates neutrophil-rich TMEs in metastasis.
17/19 This work was led by co-first authors Katey Enfield, Emma Colliver, Claudia Lee,Alastair Magness and senior authors Mihaela Angelova, Swanton Lab, Erik Sahailab and Julian Downward, The Crick.
18/19 Huge thank you to our collaborators Crispin Hiley, Sergio Quezada, James L Reading, Nicholas McGranahan, Mariam Jamal-Hanjani, UCLH, UCL Cancer Institute, The Crick, NIHR UCLH Biomedical Research, CRUK Lung Centre, PathAI and the TRACERx consortium and we gratefully acknowledge the patients and relatives in TRACERx.
19/19 This work would not have been possible without support from, Cancer Research UK,Science and Innovation at Cancer Research UK, Medical Research Council, Wellcome, The Royal Society, Horizon Europe, Marie Skłodowska Curie, European Research Council (ERC), Rosetrees Trust, The Mark Foundation for Cancer Research and Bristol Myers Squibb.”
Source: Charles Swanton/X
